Obstet Gynecol Sci.
2024 Jan;67(1):112-119. 10.5468/ogs.23120.
Association of recurrent implantation failure and recurrent pregnancy loss with peripheral blood natural killer cells and interferon-gamma level
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Fertility, Infertility and Perinatology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 2Department of Immunology, Fertility, Infertility and Perinatology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- KMID: 2550492
- DOI: http://doi.org/10.5468/ogs.23120
Abstract
Objective
Fetal uterine survival depends on maintaining an immune balance between the mother and fetus. This study aimed to investigate the correlation of blood peripheral natural killer (NK) cells and interferon-gamma (IFN-γ) with recurrent recurrent implantation failure (RIF) and recurrent pregnancy loss (RPL).
Methods
In this case-control study, peripheral blood samples were obtained from three groups of RPL, RIF, and parous women without a history of abortion or infertility problems and analyzed by lymphocyte-based flow cytometry. Afterward, the levels of NK cells and IFN-γ were determined. All data were analyzed using one-way analysis of variance and nonparametric Kruskal-Wallis tests.
Results
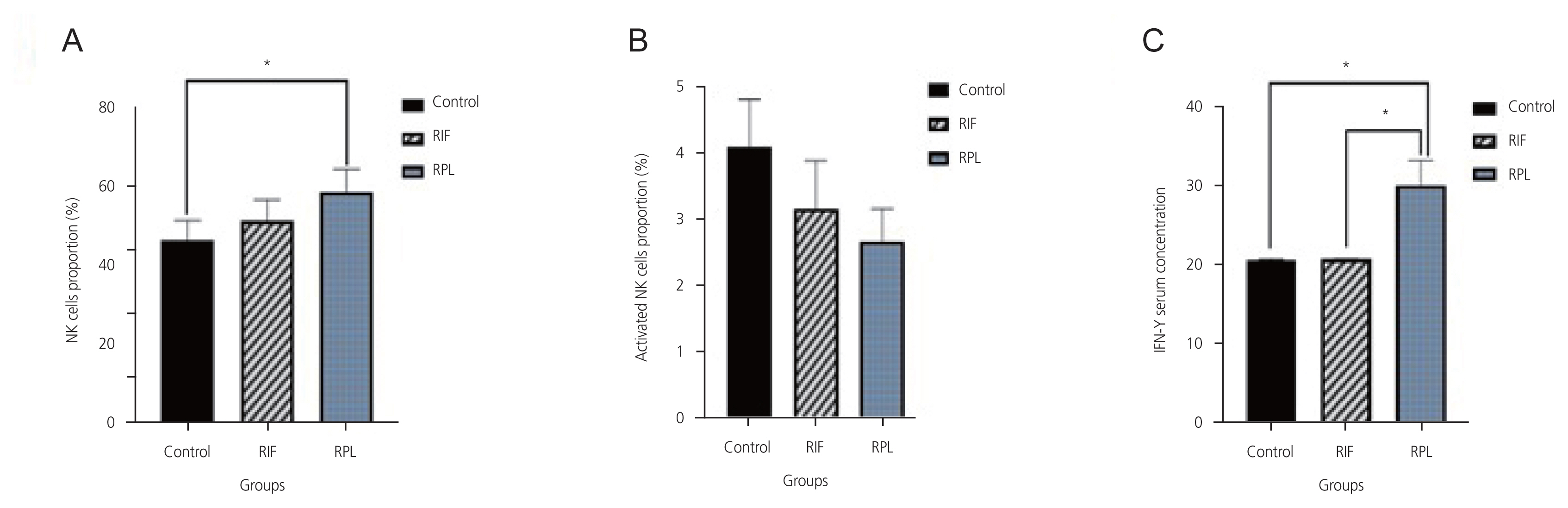
The level of IFN-γ in the RPL group was significantly higher than that in parous women and the RIF group (P<0.05), whereas its level in the RIF group was not significantly different from the control group (P>0.05). A significant correlation was found between the levels of IFN-γ and NK cells in the RPL group (r=0.481; P=0.02). However, no significant correlation was found between the levels of IFN-γ and the active NK cells in the RPL group (P=0.08). Moreover, no significant correlation was found between the levels of NK cells (whether activated or not) and IFN-γ in the RIF patients (P>0.05).
Conclusion
Immune dysfunction may not be involved in implantation failure during IVF but may be involved in recurrent miscarriage, probably by increasing IFN-γ levels.
Keyword
Figure
Reference
-
References
1. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018; 62:2–10.2. Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat Rev Dis Primers. 2020; 6:98.3. El Hachem H, Crepaux V, May-Panloup P, Descamps P, Legendre G, Bouet PE. Recurrent pregnancy loss: current perspectives. Int J Womens Health. 2017; 9:331–45.4. Scantamburlo VM, Linsingen RV, Centa LJR, Toso KFD, Scaraboto D, Araujo E Júnior, et al. Association between decreased ovarian reserve and poor oocyte quality. Obstet Gynecol Sci. 2021; 64:532–93.5. Kim SG, Paek MY, Ko IG. Peripheral blood level of natural killer cells in pregnant women with recurrent spontaneous abortion during the 6–12 weeks gestation. Arch Med Health Sci. 2019; 7:191–4.6. Zhu LY, Chen X, Xu ZZ, Xu L, Mao T, Zhang H. Changes and clinical significance of peripheral blood helper T lymphocyte and natural killer (NK) cells in unexplained recurrent spontaneous abortion (URSA) patients after abortion and successful pregnancy. Clin Exp Obstet Gynecol. 2015; 42:62–6.7. Zargar M, Pazhouhanfar R. Effects of intrauterine autologous platelet-rich plasma infusions on outcomes in women with repetitive in vitro fertilization failures: a prospective randomized study. Clin Exp Obstet Gynecol. 2021; 48:179–84.8. Genest G, Banjar S, Almasri W, Beauchamp C, Benoit J, Buckett W, et al. Immunomodulation for unexplained recurrent implantation failure: where are we now? Reproduction. 2023; 165:R39–60.9. Yuan J, Li J, Huang SY, Sun X. Characterization of the subsets of human NKT-like cells and the expression of Th1/Th2 cytokines in patients with unexplained recurrent spontaneous abortion. J Reprod Immunol. 2015; 110:81–8.10. Park S, You YA, Yun H, Choi SJ, Hwang HS, Choi SK, et al. Cervicovaginal fluid cytokines as predictive markers of preterm birth in symptomatic women. Obstet Gynecol Sci. 2020; 63:455–63.11. Iske J, Elkhal A, Tullius SG. The fetal-maternal immune interface in uterus transplantation. Trends Immunol. 2020; 41:213–24.12. Fox C, Morin S, Jeong JW, Scott RT Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertil Steril. 2016; 105:873–84.13. Sacks G, Finkelstein E. Natural killer cells and reproductive success. Am J Reprod Immunol. 2021; 85:e13291.14. Mahajan D, Sharma NR, Kancharla S, Kolli P, Tripathy A, Sharma AK, et al. Role of natural killer cells during pregnancy and related complications. Biomolecules. 2022; 12:68.15. Amand M, Iserentant G, Poli A, Sleiman M, Fievez V, Sanchez IP, et al. Human CD56dimCD16dim cells as an individualized natural killer cell subset. Front Immunol. 2017; 8:699.16. Sharma S. Natural killer cells and regulatory T cells in early pregnancy loss. Int J Dev Biol. 2014; 58:219–29.17. Amirian A, Mahani MB, Abdi F. Role of interleukin-6 (IL-6) in predicting gestational diabetes mellitus. Obstet Gynecol Sci. 2020; 63:407–16.18. Gupta S, Gaikwad HS, Nath B, Batra A. Can vitamin C and interleukin 6 levels predict preterm premature rupture of membranes: evaluating possibilities in North Indian population. Obstet Gynecol Sci. 2020; 63:432–9.19. Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. 2018; 49:397–412.20. Choghakabodi PM, Pouladzadeh M, Haybar H, Keikhaei B, Hossein Nezhad K, Jalali Far MA, et al. Biological whistleblowers for silent myocardial ischemia: diagnostic and prognostic approach. Recenti Prog Med. 2020; 111:415–25.21. Mahy Brian WJ, vanRegenmortel Marc HV. Desk encyclopedia of general virology. Griffin DE, editor. Cytokines and chemokines. 3rd ed. Atlanta: Academic Press;2008. p. 620–4.22. Ghafourian M, Karami N, Khodadadi A, Nikbakht R. Increase of CD69, CD161 and CD94 on NK cells in women with recurrent spontaneous abortion and in vitro fertilization failure. Iran J Immunol. 2014; 11:84–96.23. Bashiri A, Halper KI, Orvieto R. Recurrent implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018; 16:121.24. Armitage P, Berry G, Matthews JNS. Statistical methods in medical research. 4th ed. London: John Wiley & Sons;2002.25. Zhang H, Huang C, Chen X, Li L, Liu S, Li Y, et al. The number and cytotoxicity and the expression of cytotoxicity-related molecules in peripheral natural killer (NK) cells do not predict the repeated implantation failure (RIF) for the in vitro fertilization patients. Genes Dis. 2019; 7:283–9.26. Thum MY, Bhaskaran S, Bansal AS, Shehata H, Ford B, Sumar N, et al. Simple enumerations of peripheral blood natural killer (CD56+ NK) cells, B cells and T cells have no predictive value in IVF treatment outcome. Hum Reprod. 2005; 20:1272–6.27. Tuckerman E, Mariee N, Prakash A, Li TC, Laird S. Uterine natural killer cells in peri-implantation endometrium from women with repeated implantation failure after IVF. J Reprod Immunol. 2010; 87:60–6.28. Mardanian F, Kazeroonizadeh M, Rashidi B. Evaluation of CD56(dim) and CD56(bright) natural killer cells in peripheral blood of women with IVF failures. Iran J Reprod Med. 2015; 13:577–82.29. Dons’koi BV, Osypchuk DV, Chernyshov VP, Khazhylenko KG. Expression of natural cytotoxicity receptor NKp46 on peripheral blood natural killer cells in women with a history of recurrent implantation failures. J Obstet Gynaecol Res. 2021; 47:1009–15.30. Kuon RJ, Vomstein K, Weber M, Müller F, Seitz C, Wallwiener S, et al. The “killer cell story” in recurrent miscarriage: association between activated peripheral lymphocytes and uterine natural killer cells. J Reprod Immunol. 2017; 119:9–14.31. Kwak-Kim JYH, Gilman-Sachs A, Kim CE. T helper 1 and 2 immune responses in relationship to pregnancy, nonpregnancy, recurrent spontaneous abortions and infertility of repeated implantation failures. Chem Immunol Allergy. 2005; 88:64–79.32. Piccinni MP. T cells in normal pregnancy and recurrent pregnancy loss. Reprod Biomed Online. 2007; 14:Spec 1. 95–9.33. Shimada S, Kato EH, Morikawa M, Iwabuchi K, Nishida R, Kishi R, et al. No difference in natural killer or natural killer T-cell population, but aberrant T-helper cell population in the endometrium of women with repeated miscarriage. Hum Reprod. 2004; 19:1018–24.34. Schoenborn JR, Wilson CB. Regulation of interferongamma during innate and adaptive immune responses. Adv Immunol. 2007; 96:41–101.35. Mikhailova VA, Khokhlova EV, Bazhenov DO, Agnaeva AO, Kozyreva AR, Bespalova ON, et al. Changes in expression of Ki-67, CD16 and CD56 by natural killer cells from peripheral blood mononuclear cells in the setting of recurrent miscarriage after in vitro culturing in the presence of trophoblast cells and IL-2. Cytotechnology. 2019; 71:861–71.36. Yougbaré I, Tai WS, Zdravic D, Oswald BE, Lang S, Zhu G, et al. Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia. Nat Commun. 2017; 8:224.37. Zhu L, Aly M, Kuon RJ, Toth B, Wang H, Karakizlis H, et al. Patients with idiopathic recurrent miscarriage have abnormally high TGFß+ blood NK, NKT and T cells in the presence of abnormally low TGFß plasma levels. BMC Immunol. 2019; 20:10.38. Fiuza-Luces C, Padilla JR, Valentín J, Santana-Sosa E, Santos-Lozano A, Sanchis-Gomar F, et al. Effects of exercise on the immune function of pediatric patients with solid tumors: insights from the PAPEC randomized trial. Am J Phys Med Rehabil. 2017; 96:831–7.39. Gustafson MP, DiCostanzo AC, Wheatley CM, Kim CH, Bornschlegl S, Gastineau DA, et al. A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J Immunother Cancer. 2017; 5:30.40. Cossarizza A, Chang HD, Radbruch A, Acs A, Adam D, Adam-Klages S, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur J Immunol. 2019; 49:1457–973.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of natural killer cell number and cytolytic activity during first trimester of pregnancy in recurrent spontaneous abortion patients and fertile control
- The Preconceptional Level of Peripheral Natural Killer Cells which was Expected to Bring Successful Treatment Outcome using Low-dose Intravenous Gamma Immunoglobulin (IVIg) Infusion in Patients with Recurrent Spontaneous Abortion
- Pathogenetic factors involved in recurrent pregnancy loss from multiple aspects
- Role of endometrial immune cells in implantation
- Immunologic Effect of Gamma Interferon on Human Melanoma Cell Lime A - 375 - With Special Emphasis on Cytolytic Activity , Antiproliferative Activity and HLA Antigen Expression