Obstet Gynecol Sci.
2024 Jan;67(1):30-48. 10.5468/ogs.23193.
A glance into the roles of microRNAs (exosomal and non-exosomal) in polycystic ovary syndrome
- Affiliations
-
- 1Department of Medical Genetics, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
- 2Department of Genetics, Faculty of Basic Sciences, Shahrekord University, Shahrekord, Iran
- 3Department of Obstetrics and Gynecology, School of Medicine, Bam University of Medical Sciences, Bam, Iran
- KMID: 2550484
- DOI: http://doi.org/10.5468/ogs.23193
Abstract
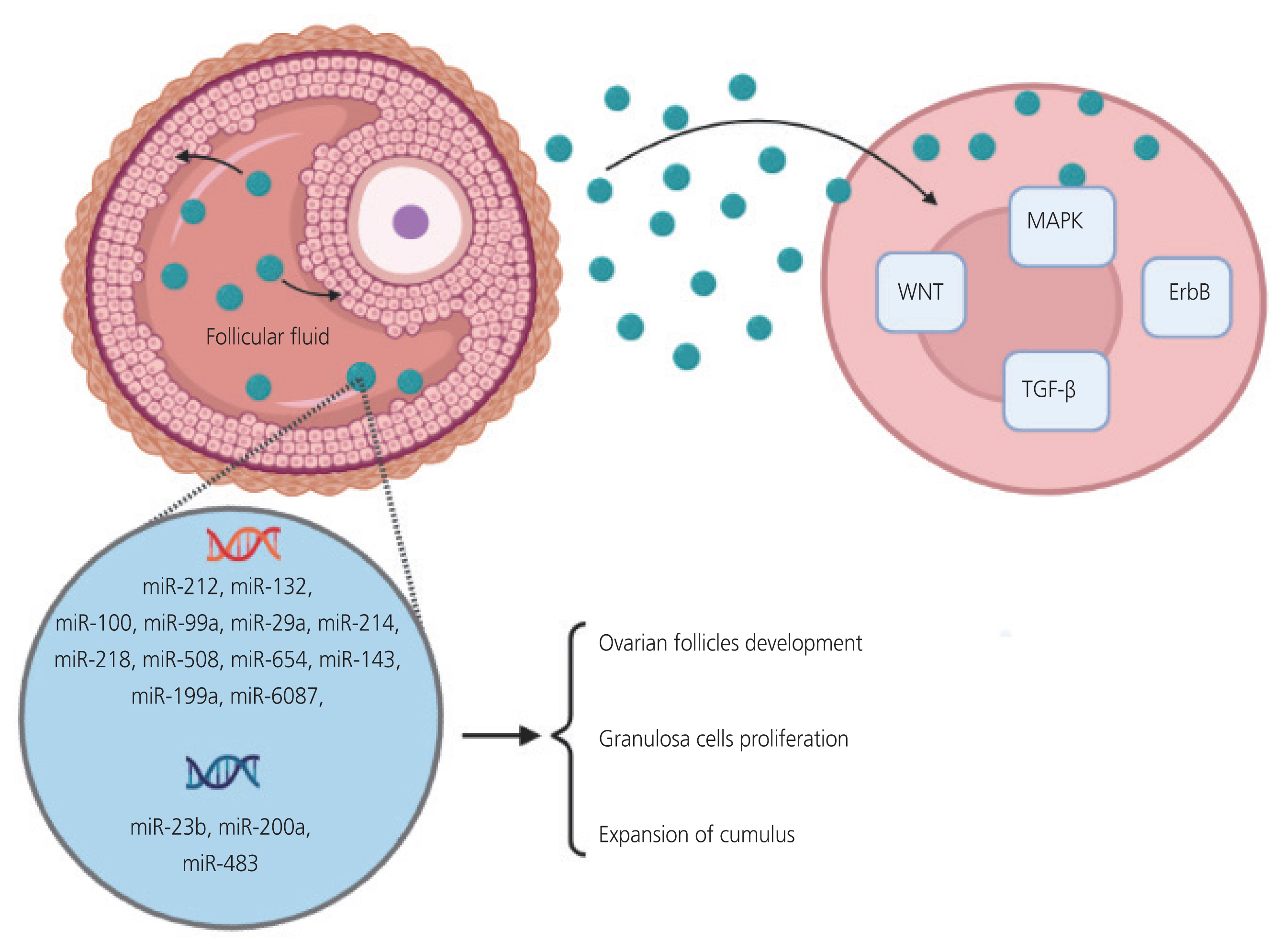
- Polycystic ovarian syndrome (PCOS) is a common endocrine disorder in women of reproductive age. The clinical symptoms include hyperandrogenism, chronic anovulation, and multiple ovarian cysts. PCOS is strongly associated with obesity and insulin resistance. MicroRNAs (miRNAs) are a group of short non-coding RNAs that play a role in the post-transcriptional regulation of gene expression and translational inhibition. They play a vital role in the regulation of multiple metabolic and hormonal processes as well as in oocyte maturation and folliculogenesis in the female reproductive system. miRNAs can be used as diagnostic biomarkers or therapeutic targets because of their stability. The encapsulation of miRNAs in extracellular vesicles or exosomes contributes to their stability. Exosomes are constantly secreted by many cells and size of about 30 to 150 nm. Enveloping miRNAs exosomes can release them for cellular communication. The induced transfer of miRNAs by exosomes is a novel process of genetic exchange between cells. Many studies have shown that along with non-exosomal miRNAs, different types of exosomal miRNAs derived from the serum and follicular fluid can play an essential role in PCOS pathogenesis. These miRNAs are involved in follicular development and various functions in granulosa cells, apoptosis, cell proliferation, and follicular atresia. The present study aimed to comprehensively review the evidence on miRNAs and their affected pathways under both non-exosomal and exosomal circumstances, primarily focusing on the pathogenesis of PCOS.
Figure
Reference
-
References
1. Ambros V. The functions of animal microRNAs. Nature. 2004; 431:350–5.2. Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol. 2017; 1509:1–10.3. Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003; 425:415–9.4. Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004; 303:95–8.5. Kirstein N, Dokaneheifard S, Cingaram PR, Valencia MG, Beckedorff F, Gomes Dos Santos H, et al. The integrator complex regulates microRNA abundance through RISC loading. Sci Adv. 2023; 9:eadf0597.6. Martinez J, Patkaniowska A, Urlaub H, Lührmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002; 110:563–74.7. Schwarz DS, Hutvágner G, Haley B, Zamore PD. Evidence that siRNAs function as guides, not primers, in the Drosophila and human RNAi pathways. Mol Cell. 2002; 10:537–48.8. Michlewski G, Cáceres JF. Post-transcriptional control of miRNA biogenesis. RNA. 2019; 25:1–16.9. Castellano L, Stebbing J. Deep sequencing of small RNAs identifies canonical and non-canonical miRNA and endogenous siRNAs in mammalian somatic tissues. Nucleic Acids Res. 2013; 41:3339–51.10. Achkar NP, Cambiagno DA, Manavella PA. miRNA biogenesis: a dynamic pathway. Trends Plant Sci. 2016; 21:1034–44.11. Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007; 26:745–52.12. Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008; 9:839–45.13. Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A. 2008; 105:5166–71.14. Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009; 136:26–36.15. Taheri F, Ebrahimi SO, Shareef S, Reiisi S. Regulatory and immunomodulatory role of miR-34a in T cell immunity. Life Sci. 2020; 262:118209.16. Li Y, Fang Y, Liu Y, Yang X. MicroRNAs in ovarian function and disorders. J Ovarian Res. 2015; 8:51.17. Chen B, Xu P, Wang J, Zhang C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene. 2019; 706:91–6.18. McGinnis LK, Luense LJ, Christenson LK. MicroRNA in ovarian biology and disease. Cold Spring Harb Perspect Med. 2015; 5:a022962.19. Yang X, Zhou Y, Peng S, Wu L, Lin HY, Wang S, et al. Differentially expressed plasma microRNAs in premature ovarian failure patients and the potential regulatory function of mir-23a in granulosa cell apoptosis. Reproduction. 2012; 144:235–44.20. Ebrahimi SO, Reiisi S, Parchami Barjui S. Increased risk of polycystic ovary syndrome (PCOS) associated with CC genotype of miR-146a gene variation. Gynecol Endocrinol. 2018; 34:793–7.21. Jiang L, Huang J, Li L, Chen Y, Chen X, Zhao X, et al. MicroRNA-93 promotes ovarian granulosa cells proliferation through targeting CDKN1A in polycystic ovarian syndrome. J Clin Endocrinol Metab. 2015; 100:E729–38.22. Naz MSG, Rahnemaei FA, Tehrani FR, Sayehmiri F, Ghasemi V, Banaei M, et al. Possible cognition changes in women with polycystic ovary syndrome: a narrative review. Obstet Gynecol Sci. 2023; 66:347–63.23. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and Pcos Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome--part 1. Endocr Pract. 2015; 21:1291–300.24. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010; 25:544–51.25. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011; 7:219–31.26. Corbett S, Morin-Papunen L. The polycystic ovary syndrome and recent human evolution. Mol Cell Endocrinol. 2013; 373:39–50.27. de Wilde MA, Goverde AJ, Veltman-Verhulst SM, Eijkemans MJ, Franx A, Fauser BC, et al. Insulin action in women with polycystic ovary syndrome and its relation to gestational diabetes. Hum Reprod. 2015; 30:1447–53.28. Dumont A, Robin G, Catteau-Jonard S, Dewailly D. Role of anti-Müllerian hormone in pathophysiology, diagnosis and treatment of polycystic ovary syndrome: a review. Reprod Biol Endocrinol. 2015; 13:137.29. Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2013; 373:29–38.30. Casarini L, Brigante G. The polycystic ovary syndrome evolutionary paradox: a genome-wide association studies-based, in silico, evolutionary explanation. J Clin Endocrinol Metab. 2014; 99:E2412–20.31. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015; 36:487–525.32. Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018; 182:27–36.33. Imbar T, Eisenberg I. Regulatory role of microRNAs in ovarian function. Fertil Steril. 2014; 101:1524–30.34. Lomelí H, Ramos-Mejía V, Gertsenstein M, Lobe CG, Nagy A. Targeted insertion of Cre recombinase into the TNAP gene: excision in primordial germ cells. Genesis. 2000; 26:116–7.35. Baley J, Li J. MicroRNAs and ovarian function. J Ovarian Res. 2012; 5:8.36. Ebrahimi SO, Reiisi S. Downregulation of miR-4443 and miR-5195-3p in ovarian cancer tissue contributes to metastasis and tumorigenesis. Arch Gynecol Obstet. 2019; 299:1453–8.37. Cai S, Chen R, Li X, Cai Y, Ye Z, Li S, et al. Downregulation of microRNA-23a suppresses prostate cancer metastasis by targeting the PAK6-LIMK1 signaling pathway. Oncotarget. 2015; 6:3904–17.38. Khan HA, Zhao Y, Wang L, Li Q, Du YA, Dan Y, et al. Identification of miRNAs during mouse postnatal ovarian development and superovulation. J Ovarian Res. 2015; 8:44.39. Sun XF, Li YP, Pan B, Wang YF, Li J, Shen W. Molecular regulation of miR-378 on the development of mouse follicle and the maturation of oocyte in vivo. Cell Cycle. 2018; 17:2230–42.40. Donadeu FX, Schauer SN, Sontakke SD. Involvement of miRNAs in ovarian follicular and luteal development. J Endocrinol. 2012; 215:323–34.41. McBride D, Carré W, Sontakke SD, Hogg CO, Law A, Donadeu FX, et al. Identification of miRNAs associated with the follicular-luteal transition in the ruminant ovary. Reproduction. 2012; 144:221–33.42. Real FM, Sekido R, Lupiáñez DG, Lovell-Badge R, Jiménez R, Burgos M. A microRNA (mmu-miR-124) prevents Sox9 expression in developing mouse ovarian cells. Biol Reprod. 2013; 89:78.43. Ro S, Song R, Park C, Zheng H, Sanders KM, Yan W. Cloning and expression profiling of small RNAs expressed in the mouse ovary. RNA. 2007; 13:2366–80.44. Zhang J, Ji X, Zhou D, Li Y, Lin J, Liu J, et al. miR-143 is critical for the formation of primordial follicles in mice. Front Biosci (Landmark Ed). 2013; 18:588–97.45. Zhang Q, Sun H, Jiang Y, Ding L, Wu S, Fang T, et al. MicroRNA-181a suppresses mouse granulosa cell proliferation by targeting activin receptor IIA. PLoS One. 2013; 8:e59667.46. Moreno JM, Núñez MJ, Quiñonero A, Martínez S, de la Orden M, Simón C, et al. Follicular fluid and mural granulosa cells microRNA profiles vary in in vitro fertilization patients depending on their age and oocyte maturation stage. Fertil Steril. 2015; 104:1037–46e1.47. Shinoda G, De Soysa TY, Seligson MT, Yabuuchi A, Fujiwara Y, Huang PY, et al. Lin28a regulates germ cell pool size and fertility. Stem Cells. 2013; 31:1001–9.48. Flemr M, Moravec M, Libova V, Sedlacek R, Svoboda P. Lin28a is dormant, functional, and dispensable during mouse oocyte-to-embryo transition. Biol Reprod. 2014; 90:131.49. Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007; 67:8433–8.50. Carletti MZ, Fiedler SD, Christenson LK. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol Reprod. 2010; 83:286–95.51. Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008; 18:997–1006.52. Mori MA, Ludwig RG, Garcia-Martin R, Brandão BB, Kahn CR. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019; 30:656–73.53. Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010; 107:810–7.54. Long W, Zhao C, Ji C, Ding H, Cui Y, Guo X, et al. Characterization of serum microRNAs profile of PCOS and identification of novel non-invasive biomarkers. Cell Physiol Biochem. 2014; 33:1304–15.55. Lanthier N, Leclercq IA. Adipose tissues as endocrine target organs. Best Pract Res Clin Gastroenterol. 2014; 28:545–58.56. Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, et al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013; 98:3068–79.57. Sirotkin AV, Lauková M, Ovcharenko D, Brenaut P, Mlyncek M. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J Cell Physiol. 2010; 223:49–56.58. Chen YH, Heneidi S, Lee JM, Layman LC, Stepp DW, Gamboa GM, et al. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes. 2013; 62:2278–86.59. Murri M, Insenser M, Fernández-Durán E, SanMillán JL, Escobar-Morreale HF. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. J Clin Endocrinol Metab. 2013; 98:E1835–44.60. Mu J, Li Q. Anomalous expression of miR-103 in polycystic ovary syndrome influenced by hormonal, and metabolic variables. Exp Mol Pathol. 2020; 116:104482.61. Xiong W, Lin Y, Xu L, Tamadon A, Zou S, Tian F, et al. Circulatory microRNA 23a and microRNA 23b and polycystic ovary syndrome (PCOS): the effects of body mass index and sex hormones in an Eastern Han Chinese population. J Ovarian Res. 2017; 10:10.62. Sathyapalan T, David R, Gooderham NJ, Atkin SL. Increased expression of circulating miRNA-93 in women with polycystic ovary syndrome may represent a novel, non-invasive biomarker for diagnosis. Sci Rep. 2015; 5:16890.63. Rashad NM, Ateya MA, Saraya YS, Elnagar WM, Helal KF, Lashin ME, et al. Association of miRNA - 320 expression level and its target gene endothelin-1 with the susceptibility and clinical features of polycystic ovary syndrome. J Ovarian Res. 2019; 12:39.64. Deswal R, Dang AS. Dissecting the role of micro-RNAs as a diagnostic marker for polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. 2020; 113:661–9e2.65. Yuan WN, Tan L. MicroRNA-320 inhibits insulin resistance in patients with PCOS through regulating ERK1/2 signaling pathway. Biomed Res. 2017; 28:4946–9.66. Flammer J, Konieczka K. Retinal venous pressure: the role of endothelin. EPMA J. 2015; 6:21.67. Jiang L, Li W, Wu M, Cao S. Ciculating miRNA-21 as a biomarker predicts polycystic ovary syndrome (PCOS) in patients. Clin Lab. 2015; 61:1009–15.68. Song DK, Sung YA, Lee H. The role of serum microRNA-6767-5p as a biomarker for the diagnosis of polycystic ovary syndrome. PLoS One. 2016; 11:e0163756.69. Li Y, Xiang Y, Song Y, Wan L, Yu G, Tan L. Dysregulated miR-142, -33b and -423 in granulosa cells target TGFBR1 and SMAD7: a possible role in polycystic ovary syndrome. Mol Hum Reprod. 2019; 25:638–46.70. Luense LJ, Veiga-Lopez A, Padmanabhan V, Christenson LK. Developmental programming: gestational testosterone treatment alters fetal ovarian gene expression. Endocrinology. 2011; 152:4974–83.71. Zhang Y, Xu L. Preliminary study of Yulin mixture affecting the miR-320/SF-1/Cyp19a1 on mouse polycystic ovary syndrome model. Gynecol Endocrinol. 2021; 37:546–53.72. Xiang Y, Song Y, Li Y, Zhao D, Ma L, Tan L. miR-483 is down-regulated in polycystic ovarian syndrome and inhibits KGN cell proliferation via targeting insulin-like growth factor 1 (IGF1). Med Sci Monit. 2016; 22:3383–93.73. Yang Y, Jiang H, Xiao L, Yang X. MicroRNA-33b-5p is overexpressed and inhibits GLUT4 by targeting HMGA2 in polycystic ovarian syndrome: an in vivo and in vitro study. Oncol Rep. 2018; 39:3073–85.74. Butler AE, Ramachandran V, Hayat S, Dargham SR, Cunningham TK, Benurwar M, et al. Expression of microRNA in follicular fluid in women with and without PCOS. Sci Rep. 2019; 9:16306.75. Sørensen AE, Wissing ML, Salö S, Englund AL, Dalgaard LT. MicroRNAs related to polycystic ovary syndrome (PCOS). Genes (Basel). 2014; 5:684–708.76. Scalici E, Traver S, Mullet T, Molinari N, Ferrières A, Brunet C, et al. Circulating microRNAs in follicular fluid, powerful tools to explore in vitro fertilization process. Sci Rep. 2016; 6:24976.77. Roth LW, McCallie B, Alvero R, Schoolcraft WB, Minjarez D, Katz-Jaffe MG. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J Assist Reprod Genet. 2014; 31:355–62.78. Naji M, Nekoonam S, Aleyasin A, Arefian E, Mahdian R, Azizi E, et al. Expression of miR-15a, miR-145, and miR-182 in granulosa-lutein cells, follicular fluid, and serum of women with polycystic ovary syndrome (PCOS). Arch Gynecol Obstet. 2018; 297:221–31.79. Cai G, Ma X, Chen B, Huang Y, Liu S, Yang H, et al. MicroRNA-145 negatively regulates cell proliferation through targeting IRS1 in isolated ovarian granulosa cells from patients with polycystic ovary syndrome. Reprod Sci. 2017; 24:902–10.80. Xu B, Zhang YW, Tong XH, Liu YS. Characterization of microRNA profile in human cumulus granulosa cells: Identification of microRNAs that regulate Notch signaling and are associated with PCOS. Mol Cell Endocrinol. 2015; 404:26–36.81. Lu J, Zhang C, Gu B, Zhang S, Geng J, Chen Y, et al. MicroRNA-182 inhibits rat ovarian granulosa cell apoptosis by targeting Smad7 in polycystic ovarian syndrome. Int J Clin Exp Pathol. 2017; 10:1380–7.82. Xue Y, Lv J, Xu P, Gu L, Cao J, Xu L, et al. Identification of microRNAs and genes associated with hyperandrogenism in the follicular fluid of women with polycystic ovary syndrome. J Cell Biochem. 2018; 119:3913–21.83. Wang M, Liu M, Sun J, Jia L, Ma S, Gao J, et al. MicroRNA-27a-3p affects estradiol and androgen imbalance by targeting Creb1 in the granulosa cells in mouse polycytic ovary syndrome model. Reprod Biol. 2017; 17:295–304.84. Zhang CL, Wang H, Yan CY, Gao XF, Ling XJ. Deregulation of RUNX2 by miR-320a deficiency impairs steroidogenesis in cumulus granulosa cells from polycystic ovary syndrome (PCOS) patients. Biochem Biophys Res Commun. 2017; 482:1469–76.85. Chen Z, Ou H, Wu H, Wu P, Mo Z. Role of microRNA in the pathogenesis of polycystic ovary syndrome. DNA Cell Biol. 2019; 38:754–62.86. Huang X, Liu C, Hao C, Tang Q, Liu R, Lin S, et al. Identification of altered microRNAs and mRNAs in the cumulus cells of PCOS patients: miRNA-509-3p promotes oestradiol secretion by targeting MAP3K8. Reproduction. 2016; 151:643–55.87. Tu J, Cheung AH, Chan CL, Chan WY. The role of microRNAs in ovarian granulosa cells in health and disease. Front Endocrinol (Lausanne). 2019; 10:174.88. Kang L, Yang C, Wu H, Chen Q, Huang L, Li X, et al. miR-26a-5p regulates TNRC6A expression and facilitates theca cell proliferation in chicken ovarian follicles. DNA Cell Biol. 2017; 36:922–9.89. Zielak-Steciwko AE, Browne JA, McGettigan PA, Gajewska M, Dzięcioł M, Szulc T, et al. Expression of microRNAs and their target genes and pathways associated with ovarian follicle development in cattle. Physiol Genomics. 2014; 46:735–45.90. Donadeu FX, Mohammed BT, Ioannidis J. A miRNA target network putatively involved in follicular atresia. Domest Anim Endocrinol. 2017; 58:76–83.91. Yang R, Chen J, Wang L, Deng A. LncRNA BANCR participates in polycystic ovary syndrome by promoting cell apoptosis. Mol Med Rep. 2019; 19:1581–6.92. Zhang BB, Li XN, Li MX, Sun YY, Shi YX, Ma TH. miR-140-3p promotes follicle granulosa cell proliferation and steroid hormone synthesis via targeting AMH in chickens. Theriogenology. 2023; 202:84–92.93. Hossain MM, Cao M, Wang Q, Kim JY, Schellander K, Tesfaye D, et al. Altered expression of miRNAs in a dihydrotestosterone-induced rat PCOS model. J Ovarian Res. 2013; 6:36.94. Crespo RP, Bachega TASS, Mendonça BB, Gomes LG. An update of genetic basis of PCOS pathogenesis. Arch Endocrinol Metab. 2018; 62:352–61.95. De Nardo Maffazioli G, Baracat EC, Soares JM, Carvalho KC, Maciel GAR. Evaluation of circulating microRNA profiles in Brazilian women with polycystic ovary syndrome: a preliminary study. PLoS One. 2022; 17:e0275031.96. Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009; 9:4997–5000.97. Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010; 56:1733–41.98. Lässer C, Alikhani VS, Ekström K, Eldh M, Paredes PT, Bossios A, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011; 9:9.99. Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002; 360:295–305.100. Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013; 4:2980.101. Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016; 5:e19276.102. McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, et al. KRAS-MEK signaling controls ago2 sorting into exosomes. Cell Rep. 2016; 15:978–87.103. Che X, Jian F, Chen C, Liu C, Liu G, Feng W. PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J Mol Endocrinol. 2020; 64:1–12.104. Hong Y, Wu J, Yu S, Hui M, Lin S. Serum-derived exosomal microRNAs in lipid metabolism in polycystic ovary syndrome. Reprod Sci. 2022; 29:2625–35.105. Zhang F, Li SP, Zhang T, Yu B, Zhang J, Ding HG, et al. High throughput microRNAs sequencing profile of serum exosomes in women with and without polycystic ovarian syndrome. PeerJ. 2021; 9:e10998.106. Jiang X, Li J, Zhang B, Hu J, Ma J, Cui L, et al. Differential expression profile of plasma exosomal microRNAs in women with polycystic ovary syndrome. Fertil Steril. 2021; 115:782–92.107. Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, et al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013; 98:3068–79.108. Sohel MMH, Hoelker M, Schellander K, Tesfaye D. The extent of the abundance of exosomal and non-exosomal extracellular miRNAs in the bovine follicular fluid. Reprod Domest Anim. 2022; 57:1208–17.109. Li H, Huang X, Chang X, Yao J, He Q, Shen Z, et al. S100-A9 protein in exosomes derived from follicular fluid promotes inflammation via activation of NF-κB pathway in polycystic ovary syndrome. J Cell Mol Med. 2020; 24:114–25.110. Santonocito M, Vento M, Guglielmino MR, Battaglia R, Wahlgren J, Ragusa M, et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril. 2014; 102:1751–61e1.111. Hu J, Tang T, Zeng Z, Wu J, Tan X, Yan J. The expression of small RNAs in exosomes of follicular fluid altered in human polycystic ovarian syndrome. PeerJ. 2020; 8:e8640.112. Yang Q, Liu L, Huang H. Extraction and identification of exosomes in follicular fluid from patients with polycystic ovary syndrome and isolation and detection of miRNAs in exosomes. J Shanghai Jiaotong Univ (Med Sci). 2017; 38:1085–9.113. Yuan D, Luo J, Sun Y, Hao L, Zheng J, Yang Z. PCOS follicular fluid derived exosomal miR-424-5p induces granulosa cells senescence by targeting CDCA4 expression. Cell Signal. 2021; 85:110030.114. Zhao Y, Pan S, Li Y, Wu X. Exosomal miR-143-3p derived from follicular fluid promotes granulosa cell apoptosis by targeting BMPR1A in polycystic ovary syndrome. Sci Rep. 2022; 12:4359.115. Zhou Z, Tu Z, Zhang J, Tan C, Shen X, Wan B, et al. Follicular fluid-derived exosomal microRNA-18b-5p regulates PTEN-mediated PI3K/Akt/mTOR signaling pathway to inhibit polycystic ovary syndrome development. Mol Neurobiol. 2022; 59:2520–31.116. Hanna J, Hossain GS, Kocerha J. The potential for microRNA therapeutics and clinical research. Front Genet. 2019; 10:478.117. Johnson AL. Intracellular mechanisms regulating cell survival in ovarian follicles. Anim Reprod Sci. 2003; 78:185–201.118. Yang MY, Rajamahendran R. Morphological and biochemical identification of apoptosis in small, medium, and large bovine follicles and the effects of follicle-stimulating hormone and insulin-like growth factor-I on spontaneous apoptosis in cultured bovine granulosa cells. Biol Reprod. 2000; 62:1209–17.119. Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, et al. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008; 93:881–7.120. Weroha SJ, Haluska P. The insulin-like growth factor system in cancer. Endocrinol Metab Clin North Am. 2012; 41:335–50. –vi.121. Geng Y, Sui C, Xun Y, Lai Q, Jin L. MiRNA-99a can regulate proliferation and apoptosis of human granulosa cells via targeting IGF-1R in polycystic ovary syndrome. J Assist Reprod Genet. 2019; 36:211–21.122. Wang T, Liu Y, Lv M, Xing Q, Zhang Z, He X, et al. miR-323-3p regulates the steroidogenesis and cell apoptosis in polycystic ovary syndrome (PCOS) by targeting IGF-1. Gene. 2019; 683:87–100.123. Dekel N, Beers WH. Development of the rat oocyte in vitro: inhibition and induction of maturation in the presence or absence of the cumulus oophorus. Dev Biol. 1980; 75:247–54.124. Salehi E, Aflatoonian R, Moeini A, Yamini N, Asadi E, Khosravizadeh Z, et al. Apoptotic biomarkers in cumulus cells in relation to embryo quality in polycystic ovary syndrome. Arch Gynecol Obstet. 2017; 296:1219–27.125. Bakhshalizadeh S, Amidi F, Alleyassin A, Soleimani M, Shirazi R, Shabani Nashtaei M. Modulation of steroidogenesis by vitamin D3 in granulosa cells of the mouse model of polycystic ovarian syndrome. Syst Biol Reprod Med. 2017; 63:150–61.126. Yang HS, Cho MH, Zakowicz H, Hegamyer G, Sonenberg N, Colburn NH. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol. 2004; 24:3894–906.127. Ding L, Gao F, Zhang M, Yan W, Tang R, Zhang C, et al. Higher PDCD4 expression is associated with obesity, insulin resistance, lipid metabolism disorders, and granulosa cell apoptosis in polycystic ovary syndrome. Fertil Steril. 2016; 105:1330–7e3.128. Zhao Y, Tao M, Wei M, Du S, Wang H, Wang X. Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif Cells Nanomed Biotechnol. 2019; 47:3804–13.129. Wei Y, Lu S, Hu Y, Guo L, Wu X, Liu X, et al. MicroRNA-135a regulates VEGFC expression and promotes luteinized granulosa cell apoptosis in polycystic ovary syndrome. Reprod Sci. 2020; 27:1436–42.130. Bago R, Sommer E, Castel P, Crafter C, Bailey FP, Shpiro N, et al. The hVps34-SGK3 pathway alleviates sustained PI3K/Akt inhibition by stimulating mTORC1 and tumour growth. EMBO J. 2016; 35:2263.131. Wang W, Dong J, Wang M, Yao S, Tian X, Cui X, et al. miR-148a-3p suppresses epithelial ovarian cancer progression primarily by targeting c-Met. Oncol Lett. 2018; 15:6131–6.132. La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS; ESHRE Special Interest Group for Reproductive Endocrinology--AMH Round Table. Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod. 2009; 24:2264–75.133. Díaz M, Bassols J, López-Bermejo A, de Zegher F, Ibáñez L. Low circulating levels of miR-451a in girls with polycystic ovary syndrome: different effects of randomized treatments. J Clin Endocrinol Metab. 2020; 105:dgz204.134. Chen WH, Huang QY, Wang ZY, Zhuang XX, Lin S, Shi QY. Therapeutic potential of exosomes/miRNAs in polycystic ovary syndrome induced by the alteration of circadian rhythms. Front Endocrinol (Lausanne). 2022; 13:918805.135. Anna G, Kannan NN. Post-transcriptional modulators and mediators of the circadian clock. Chronobiol Int. 2021; 38:1244–61.136. Torres M, Becquet D, Franc JL, François-Bellan AM. Circadian processes in the RNA life cycle. Wiley Interdiscip Rev RNA. 2018; 9:e1467.137. Tao SC, Guo SC. Extracellular vesicles: potential participants in circadian rhythm synchronization. Int J Biol Sci. 2018; 14:1610–20.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Next-generation sequencing analysis of exosomal microRNAs: Fusobacterium nucleatum regulates the expression profiling of exosomal microRNAs in human colorectal cancer cells
- Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma
- Exosomal microRNAs (miRNAs) in blood and urine under physiological conditions: a comparative study
- Medical diagnosis and treatment of polycystic ovary syndrome
- Exosomes as the source of biomarkers of metabolic diseases