Korean J Transplant.
2023 Dec;37(4):286-292. 10.4285/kjt.23.0066.
Factors associated with operational tolerance after liver transplantation: a single center retrospective study
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2550240
- DOI: http://doi.org/10.4285/kjt.23.0066
Abstract
- Background
Liver transplantation has adverse effects from life-long immunosuppression that limit the improvement of long-term outcomes. Achieving clinical operational tolerance is a major goal in organ transplantation.
Methods
This study analyzed liver transplantation patients at a single institution from 1998 to 2020, excluding those who died within 1-year posttransplant. Operational tol-erance was defined as normal liver function even after immunosuppressive drugs were discontinued. Propensity score matching was implemented at a 1:2 ratio for the tolerant group (TG) and the nontolerant group (NTG).
Results
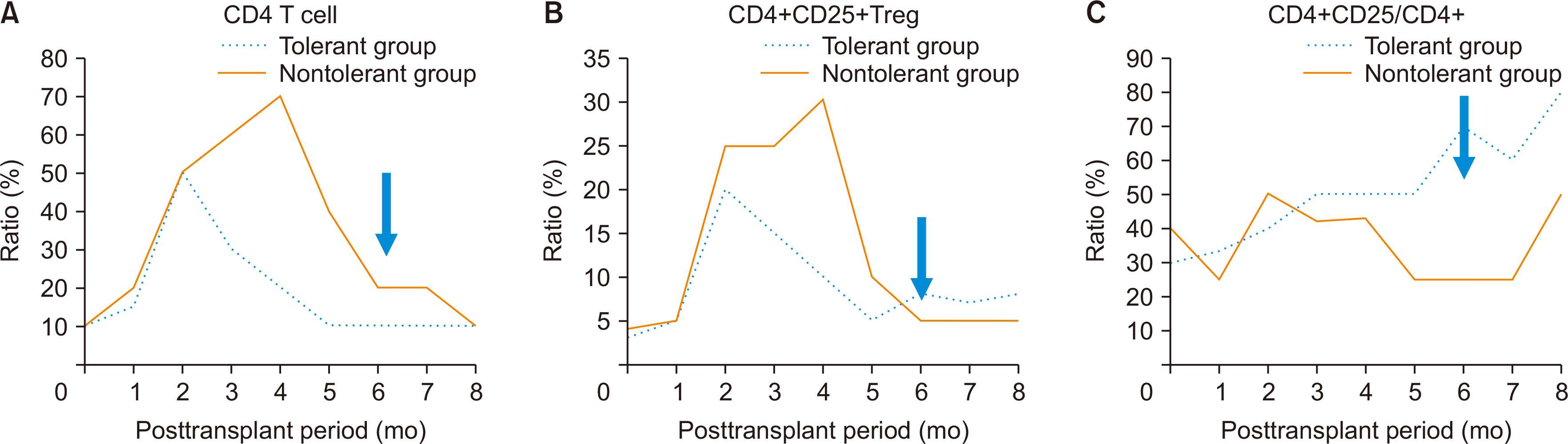
Out of 2,300 recipients, 99 achieved operational tolerance without rejection. No significant differences in sex or body mass index (BMI) were found between the TG and NTG. There was a significantly higher percentage of children in the TG (24.0%) than in the NTG (10.1%). The NTG had more living donor liver transplants. Among 2,054 adult recipients, no significant differences in age, sex, or BMI were found between the TG and the NTG. However, the rate of living donor liver transplantation was 40.3% (29/75) in the TG and 84.8% in the NTG (P<0.001). The propensity score-matched analysis showed higher chronic renal failure rates and a higher graft recipient weight ratio in the TG, along with shorter warm ischemic times during surgery. After immunosuppressant withdrawal, a significant increase in the ratio of CD4+CD25+ T cells to total CD4+ T cells was observed in the TG.
Conclusions
These findings suggest that larger, healthier grafts are more conducive to inducing tolerance, and regulatory T cells are crucial in achieving tolerance.
Figure
Reference
-
1. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. 2009; Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 10:35–43. DOI: 10.1016/S1470-2045(08)70284-5. PMID: 19058754.2. Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. 2007; Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 7:2587–96. DOI: 10.1111/j.1600-6143.2007.01965.x. PMID: 17868066.3. Zhang W, Fung J. 2017; Limitations of current liver transplant immunosuppressive regimens: renal considerations. Hepatobiliary Pancreat Dis Int. 16:27–32. DOI: 10.1016/S1499-3872(16)60167-4. PMID: 28119255.4. Rodríguez-Perálvarez M, Guerrero-Misas M, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. 2017; Maintenance immunosuppression for adults undergoing liver transplantation: a network meta-analysis. Cochrane Database Syst Rev. 3:CD011639. DOI: 10.1002/14651858.CD011639.pub2. PMID: 28362060.5. Wiesner R, Rabkin J, Klintmalm G, McDiarmid S, Langnas A, Punch J, et al. 2001; A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl. 7:442–50. DOI: 10.1053/jlts.2001.23356. PMID: 11349266.6. Neuhaus P, Clavien PA, Kittur D, Salizzoni M, Rimola A, Abeywickrama K, et al. 2002; Improved treatment response with basiliximab immunoprophylaxis after liver transplantation: results from a double-blind randomized placebo-controlled trial. Liver Transpl. 8:132–42. DOI: 10.1053/jlts.2002.30302. PMID: 11862589.7. Pageaux GP, Calmus Y, Boillot O, Ducerf C, Vanlemmens C, Boudjema K, et al. 2004; Steroid withdrawal at day 14 after liver transplantation: a double-blind, placebo-controlled study. Liver Transpl. 10:1454–60. DOI: 10.1002/lt.20291. PMID: 15558584.8. Moench C, Barreiros AP, Schuchmann M, Bittinger F, Thiesen J, Hommel G, et al. 2007; Tacrolimus monotherapy without steroids after liver transplantation: a prospective randomized double-blinded placebo-controlled trial. Am J Transplant. 7:1616–23. DOI: 10.1111/j.1600-6143.2007.01804.x. PMID: 17511685.9. Lee KW, Park JB, Park H, Kwon Y, Lee JS, Kim KS, et al. 2020; Inducing transient mixed chimerism for allograft survival without maintenance immunosuppression with combined kidney and bone marrow transplantation: protocol optimization. Transplantation. 104:1472–82. DOI: 10.1097/TP.0000000000003006. PMID: 31634324.10. Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, et al. 1969; Induction of immunological tolerance by porcine liver allografts. Nature. 223:472–6. DOI: 10.1038/223472a0. PMID: 4894426.11. Kamada N, Brons G, Davies HS. 1980; Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 29:429–31. DOI: 10.1097/00007890-198005000-00021. PMID: 6990572.12. Kamada N, Davies HS, Roser B. 1981; Reversal of transplantation immunity by liver grafting. Nature. 292:840–2. DOI: 10.1038/292840a0. PMID: 7022223.13. Subbotin V, Sun H, Aitouche A, Valdivia LA, Fung JJ, Starzl TE, et al. 1997; Abrogation of chronic rejection in a murine model of aortic allotransplantation by prior induction of donor-specific tolerance. Transplantation. 64:690–5. DOI: 10.1097/00007890-199709150-00005. PMID: 9311704. PMCID: PMC2957293.14. Benítez C, Arancibia JP, Arrese M, Soza A, Domínguez P, Jarufe N, et al. 2011; Operational tolerance after liver transplantation, more common than we think: a case report. Ann Hepatol. 10:361–4. DOI: 10.1016/S1665-2681(19)31551-0. PMID: 21677341.15. Choi GS. 2014; Clinical immune tolerance in liver transplantatiom: present and future. Hanyang Med Rev. 34:197–201. DOI: 10.7599/hmr.2014.34.4.197.16. Ellias SD, Larson EL, Taner T, Nyberg SL. 2021; Cell-mediated therapies to facilitate operational tolerance in liver transplantation. Int J Mol Sci. 22:4016. DOI: 10.3390/ijms22084016. PMID: 33924646. PMCID: PMC8069094.17. Cvetkovski F, Hexham JM, Berglund E. 2021; Strategies for liver transplantation tolerance. Int J Mol Sci. 22:2253. DOI: 10.3390/ijms22052253. PMID: 33668238. PMCID: PMC7956766.18. Hann A, Oo YH, Perera MT. 2021; Regulatory T-cell therapy in liver transplantation and chronic liver disease. Front Immunol. 12:719954. DOI: 10.3389/fimmu.2021.719954. PMID: 34721383. PMCID: PMC8552037.19. Syed-Ahmed M, Narayanan M. 2019; Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. 26:8–15. DOI: 10.1053/j.ackd.2019.01.004. PMID: 30876622.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Immune Tolerance in Liver Transplantation: Present and Future
- Accidental ABO-incompatible pediatric liver transplantation with blood group antigen immune and operational tolerance: a case report with 21 years of follow-up
- Issues on Long-term Management after Liver Transplantation in Children
- Comparative Analyses of Signature Genes in Acute Rejection and Operational Tolerance
- Cellular and genetic signatures of operational tolerance in kidney transplant recipients through single cell RNA sequencing analysis