J Cerebrovasc Endovasc Neurosurg.
2023 Dec;25(4):390-402. 10.7461/jcen.2023.E2023.04.017.
Natural course of chronic subdural hematoma following surgical clipping of unruptured intracranial aneurysm by pterional approach
- Affiliations
-
- 1Department of Neurosurgery, Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea
- KMID: 2549444
- DOI: http://doi.org/10.7461/jcen.2023.E2023.04.017
Abstract
Objective
Chronic subdural hematoma (CSDH) is a neurological complication following clipping surgery. However, the natural course and ideal approach for the treatment of clipping-related-CSDH (CR-CSDH) have not been clearly established. We aimed to investigate the course of CR-CSDH using chronological radiological findings.
Methods
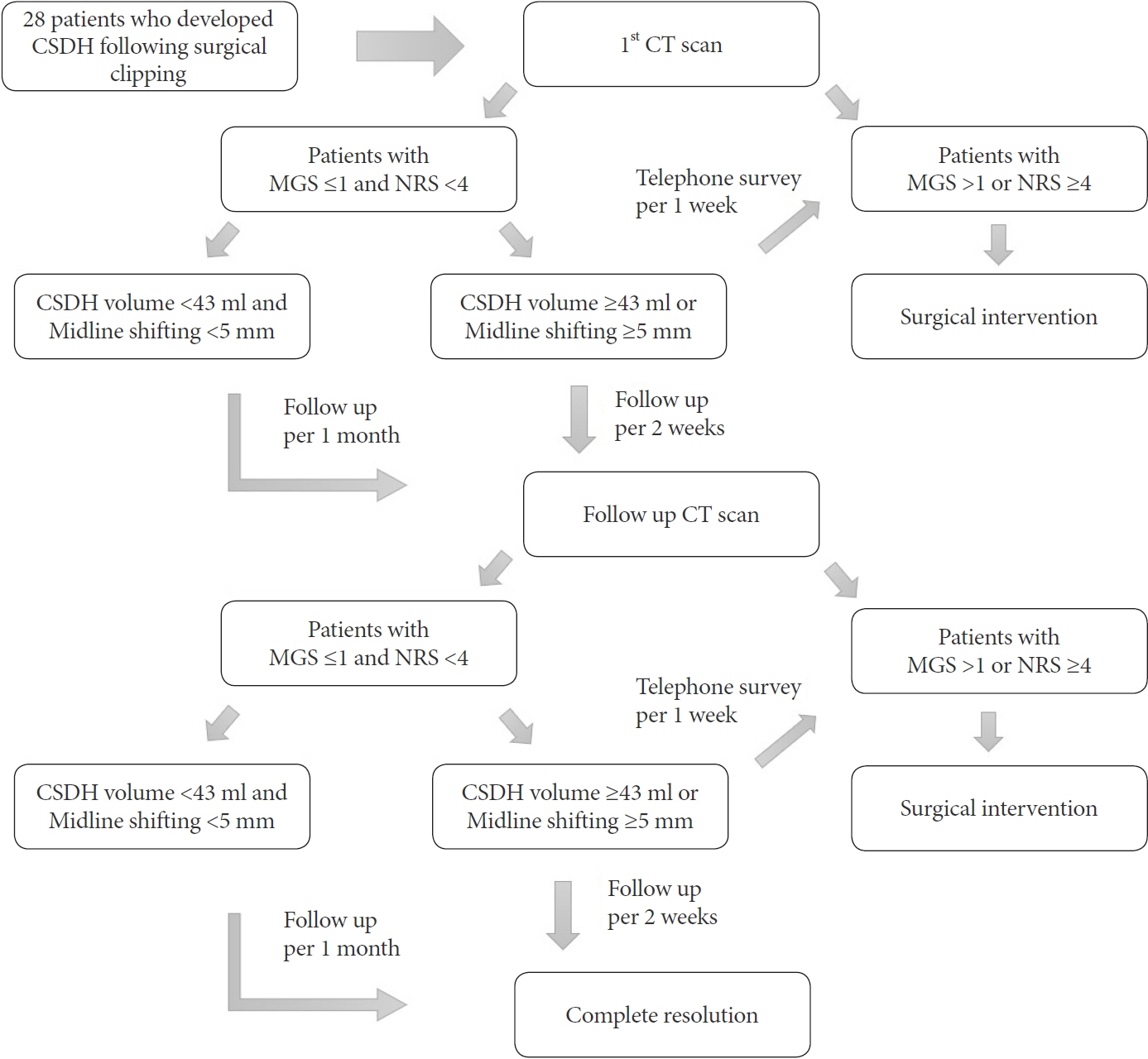
We performed a retrospective analysis of 28 (3.8%) patients who developed CSDH among 736 patients who underwent surgical clipping using pterional approach for unruptured aneurysms at our institution between December 2010 and December 2018. Patients underwent follow-up CT scan 6–8 weeks after clipping surgery and decision to pursue surgical intervention rests upon the patient’s symptom based on the Markwalder’s grading scale (MGS) and numeric rating scale (NRS).
Results
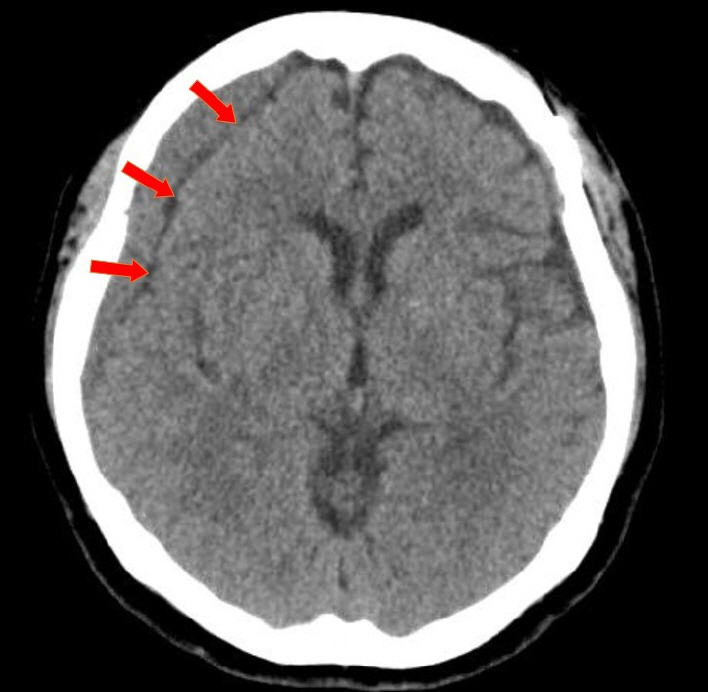
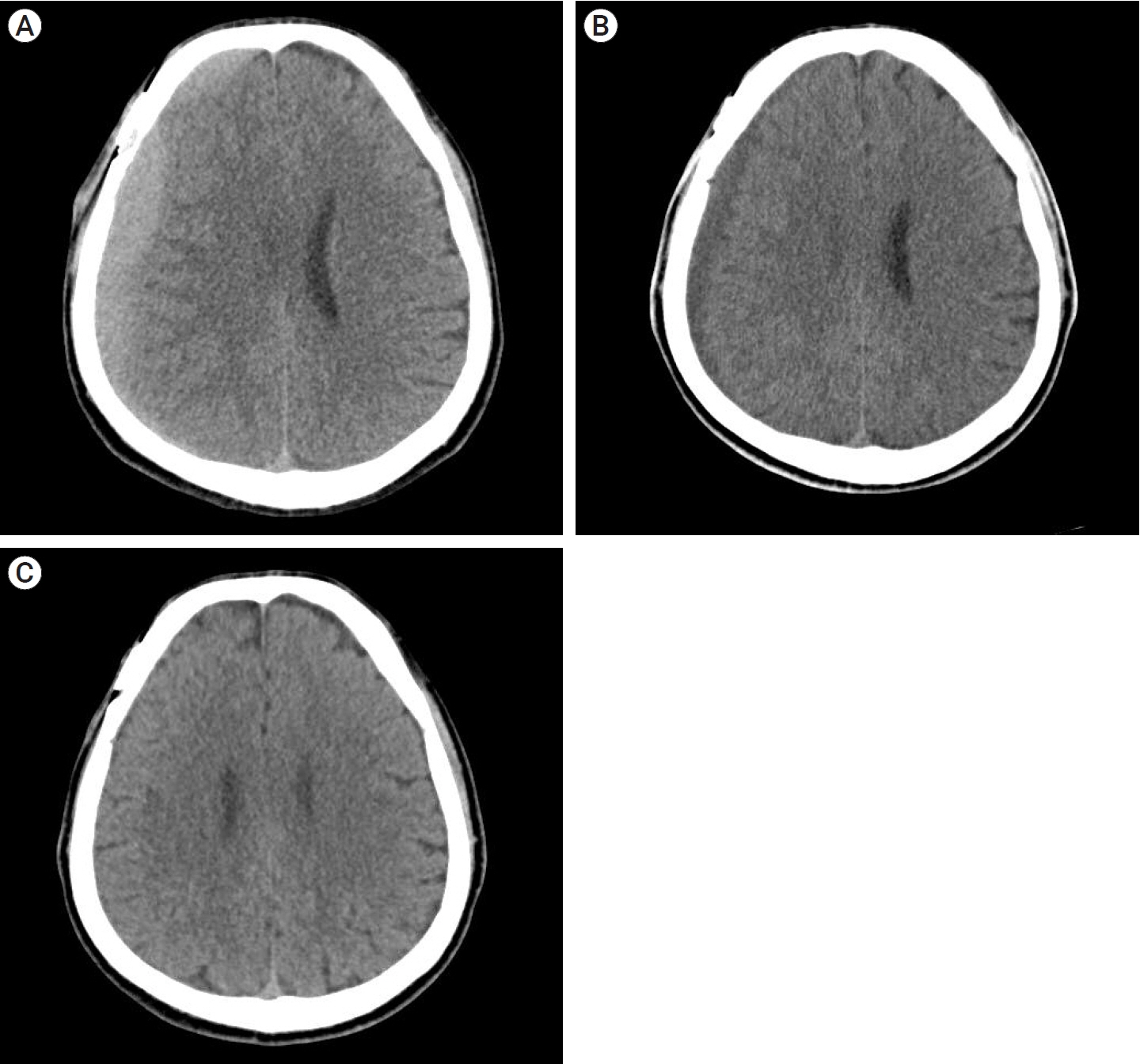
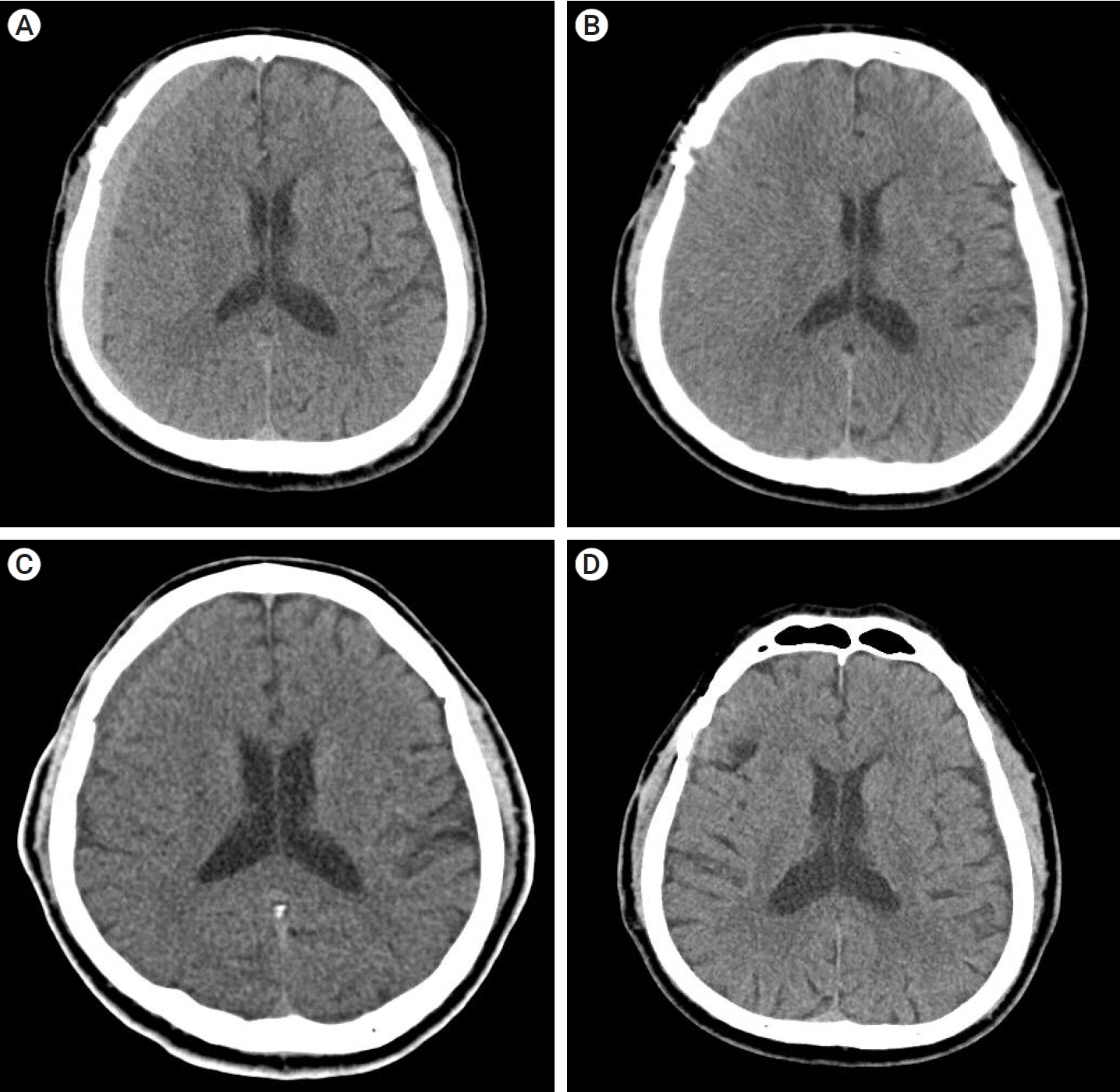
Of the 28 patients, 3 patients (10.7%) underwent surgery, while 25 (89.2%) showed spontaneous resolution of CR-CSDH. Eighteen patients (64.2%) had mild headache with MGS of 0–1. The mean maximum hematoma volume was 41.9±30.9 ml (5.8–135 ml), and 26 patients (92.8%) had homogeneous hematoma. The mean time to hematoma resolution was 126.7±52.9 days (46–228 days). Comparing group of CR-CSDH volume ≥43 ml or a midline shift ≥5 mm, the difference in presence of linear low-density area (p=0.002) and age (p=0.026) between the conservative and operative groups were found to be statistically significant.
Conclusions
Most CR-CSDH cases spontaneously resolved within 4 months. Therefore, we suggest that close observation should be performed if patient’s symptoms are mild and special radiologic findings are present, despite its relatively large volume and midline shifting.
Figure
Reference
-
1. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017; May. 14(1):108.2. Feghali J, Yang W, Huang J. Updates in chronic subdural hematoma: Epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. 2020; Sep. 141:339–45.3. Holdgate A, Asha S, Craig J, Thompson J. Comparison of a verbal numeric rating scale with the visual analogue scale for the measurement of acute pain. Emerg Med (Fremantle). 2003; Oct-Dec. 15(5-6):441–6.4. Horikoshi T, Naganuma H, Fukasawa I, Uchida M, Nukui H. Computed tomography characteristics suggestive of spontaneous resolution of chronic subdural hematoma. Neurol Med Chir (Tokyo). 1998; Sep. 38(9):527–32. discussion 532.5. Jalbert JJ, Isaacs AJ, Kamel H, Sedrakyan A. Clipping and coiling of unruptured intracranial aneurysms among medicare beneficiaries, 2000 to 2010. Stroke. 2015; Sep. 46(9):2452–7.6. Kang JH, Huh SK, Kim J, Park KY, Chung J. Subdural fluid collection after the clipping of unruptured intracranial aneurysms: Its clinical course and significance. World Neurosurg. 2018; Aug. 116:e266–72.7. Kim HC, Ko JH, Yoo DS, Lee SK. Spontaneous resolution of chronic subdural hematoma: Close observation as a treatment strategy. J Korean Neurosurg Soc. 2016; Nov. 59(6):628–36.8. Kim JH, Kim CH, Lee CY. Efficacy of arachnoid-plasty on chronic subdural hematoma following surgical clipping of unruptured intracranial aneurysms. World Neurosurg. 2017; Aug. 104:303–10.9. Kristof RA, Grimm JM, Stoffel-Wagner B. Cerebrospinal fluid leakage into the subdural space: possible influence on the pathogenesis and recurrence frequency of chronic subdural hematoma and subdural hygroma. J Neurosurg. 2008; Feb. 108(2):275–80.10. Kwon MY, Kim CH, Lee CY. Predicting factors of chronic subdural hematoma following surgical clipping in unruptured and ruptured intracranial aneurysm. J Korean Neurosurg Soc. 2016; Sep. 59(5):458–65.11. Lee KS. Natural history of chronic subdural haematoma. Brain Inj. 2004; Apr. 18(4):351–8.12. Lee WJ, Jo KI, Yeon JY, Hong SC, Kim JS. Incidence and risk factors of chronic subdural hematoma after surgical clipping for unruptured anterior circulation aneurysms. J Korean Neurosurg Soc. 2015; Apr. 57(4):271–5.13. Markwalder TM, Reulen HJ. Influence of neomembranous organisation, cortical expansion and subdural pressure on the post-operative course of chronic subdural haematoma: An analysis of 201 cases. Acta Neurochir (Wien). 1986; 79(2-4):100–6.14. Naganuma H, Fukamachi A, Kawakami M, Misumi S, Nakajima H, Wakao T. Spontaneous resolution of chronic subdural hematomas. Neurosurgery. 1986; Nov. 19(5):794–8.15. Nomura S, Kashiwagi S, Fujisawa H, Ito H, Nakamura K. Characterization of local hyperfibrinolysis in chronic subdural hematomas by SDS-PAGE and immunoblot. J Neurosurg. 1994; Dec. 81(6):910–3.16. Ohno T, Iihara K, Takahashi JC, Nakajima N, Satow T, Hishikawa T, et al. Incidence and risk factors of chronic subdural hematoma after aneurysmal clipping. World Neurosurg. 2013; Nov. 80(5):534–7.17. Park J, Cho JH, Goh DH, Kang DH, Shin IH, Hamm IS. Postoperative subdural hygroma and chronic subdural hematoma after unruptured aneurysm surgery: Age, sex, and aneurysm location as independent risk factors. J Neurosurg. 2016; Feb. 124(2):310–7.18. Parlato C, Guarracino A, Moraci A. Spontaneous resolution of chronic subdural hematoma. Surg Neurol. 2000; Apr. 53(4):312–5. discussion 315.19. Son S, Yoo CJ, Lee SG, Kim EY, Park CW, Kim WK. Natural course of initially non-operated cases of acute subdural hematoma: The risk factors of hematoma progression. J Korean Neurosurg Soc. 2013; Sep. 54(3):211–9.20. Yang W, Huang J. Chronic subdural hematoma: Epidemiology and natural history. Neurosurg Clin N Am. 2017; Apr. 28(2):205–10.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison between Lateral Supraorbital Approach and Pterional Approach in the Surgical Treatment of Unruptured Intracranial Aneurysms
- Predicting Factors of Chronic Subdural Hematoma Following Surgical Clipping in Unruptured and Ruptured Intracranial Aneurysm
- Guideline for Management of Unruptured Intracranial Aneurysms: Preliminary Report
- Modified Arachnoid Plasty Reduces Chronic Subdural Hematoma after Unruptured Aneurysm Clipping : Technical Note
- Two Cases of Posterior Communicating Artery Aneurysm Complicated by Massive Subdural Hematoma