Endocrinol Metab.
2023 Dec;38(6):782-787. 10.3803/EnM.2023.1738.
Partial Deletion of Perk Improved High-Fat Diet-Induced Glucose Intolerance in Mice
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2549271
- DOI: http://doi.org/10.3803/EnM.2023.1738
Abstract
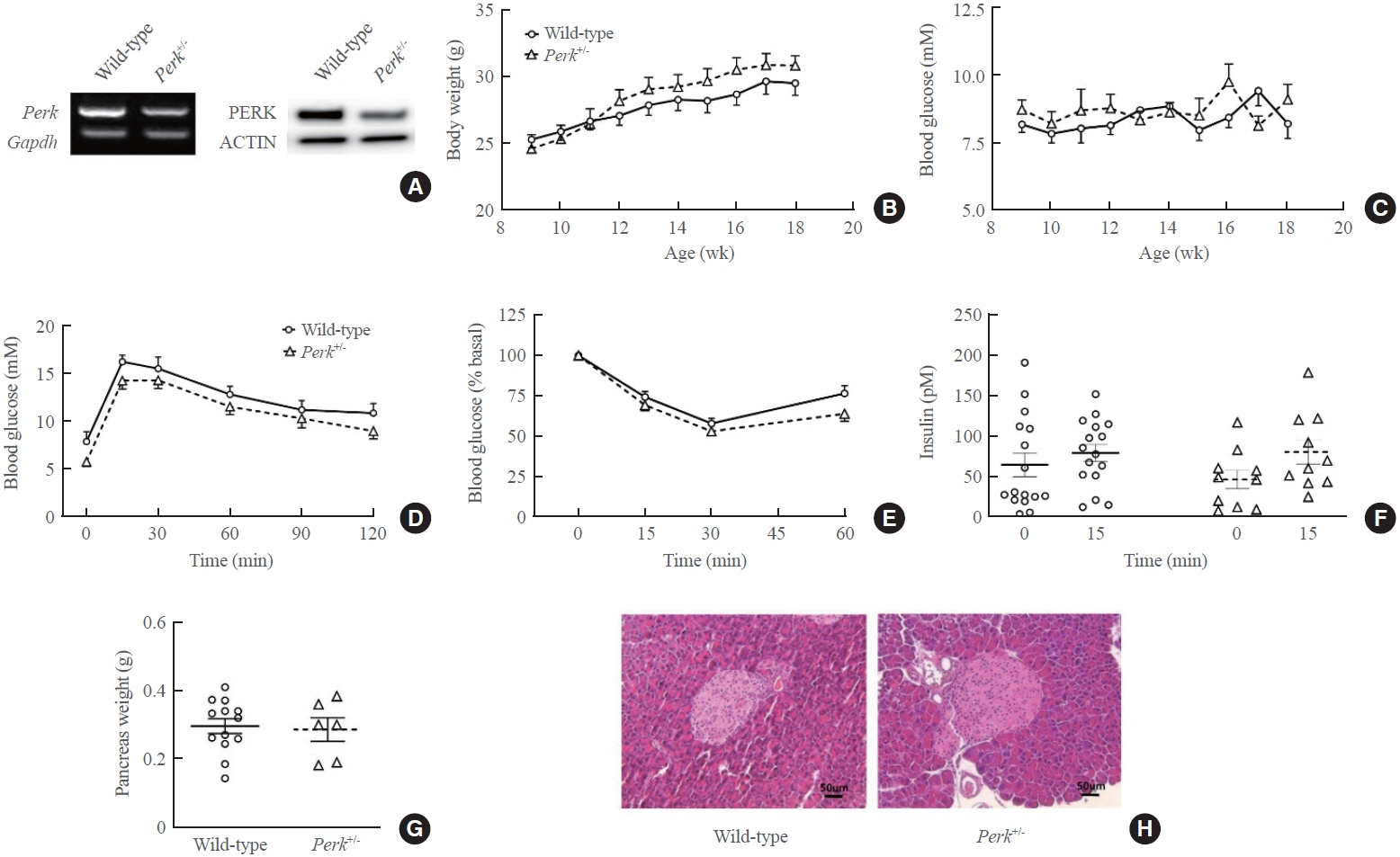
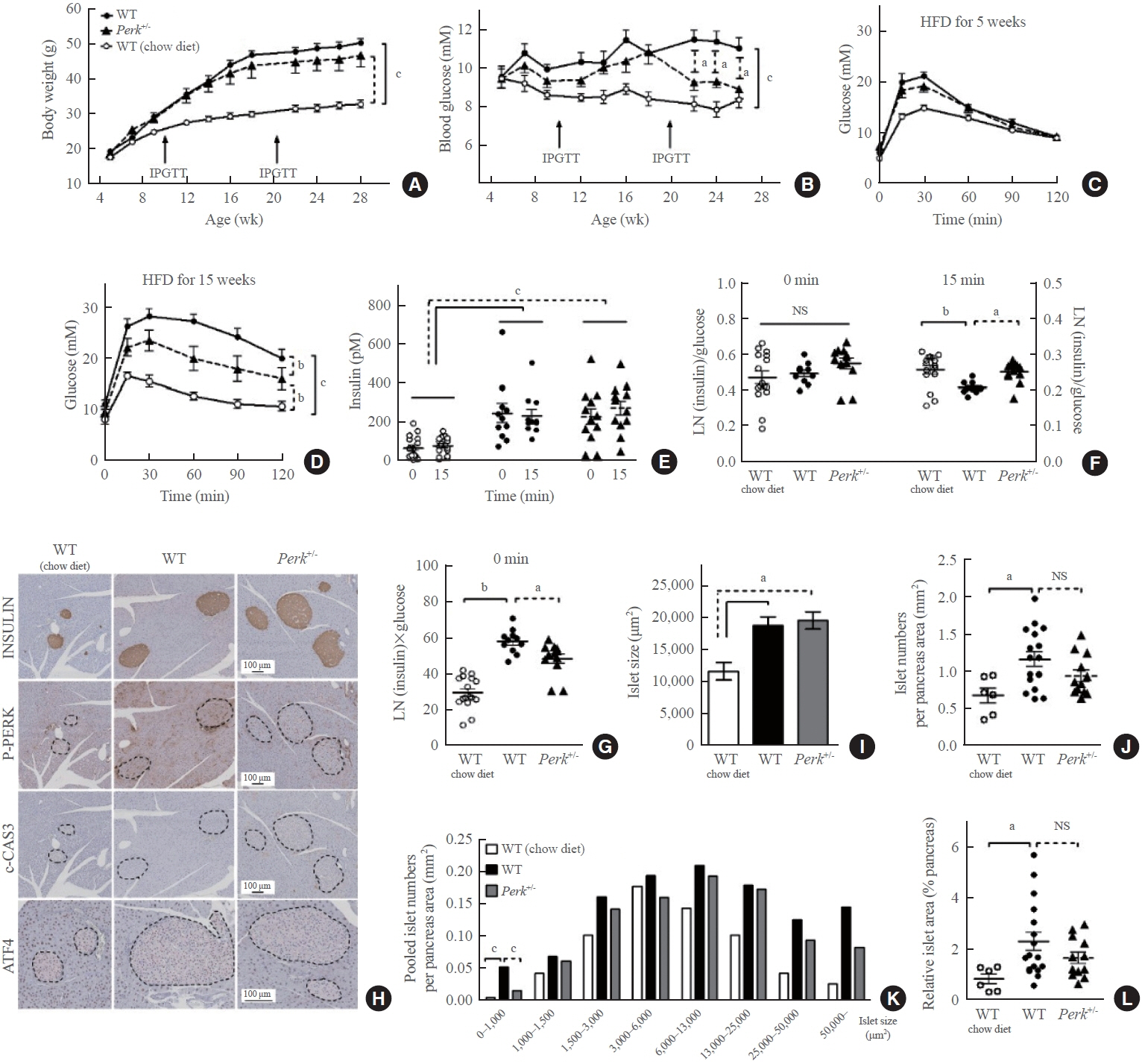
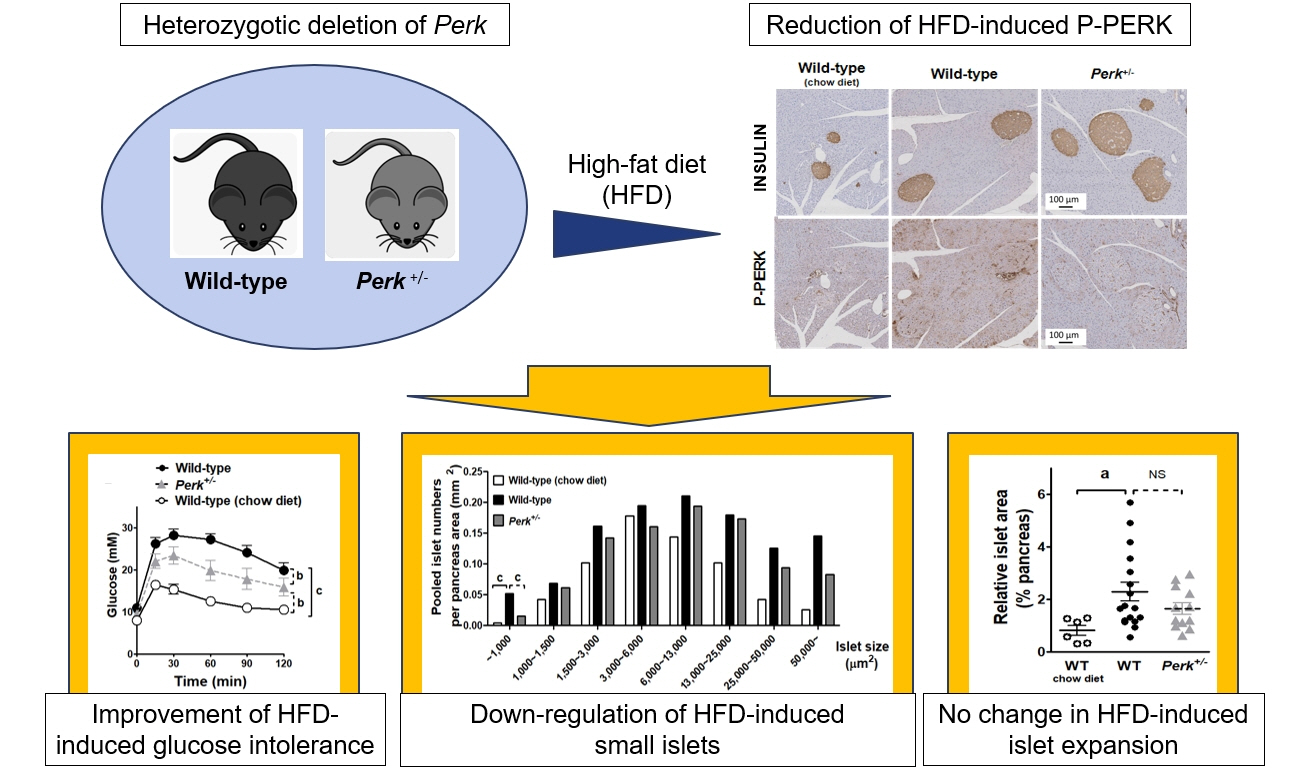
- Although pancreatic endoplasmic reticulum kinase (PERK) is indispensable to beta cells, low-dose PERK inhibitor improved glucose- stimulated insulin secretion (GSIS) and hyperglycemia in diabetic mice. Current study examined if partial deletion of Perk (Perk+/-) recapitulated the effects of PERK inhibitor, on the contrary to the complete deletion. Perk+/- mice and wild-type controls were fed with a high-fat diet (HFD) for 23 weeks. Glucose tolerance was evaluated along with serum insulin levels and islet morphology. Perk+/- mice on normal chow were comparable to wild-type mice in various metabolic features. HFD-induced obesity was not influenced by Perk reduction; however, HFD-induced glucose intolerance was significantly improved since 15-week HFD. HFD-induced compromises in GSIS were relieved by Perk reduction, accompanied by reductions in phosphorylated PERK and activating transcription factor 4 (ATF4) in the islets. Meanwhile, HFD-induced islet expansion was not significantly affected. In summary, partial deletion of Perk improved glucose tolerance and GSIS impaired by diet-induced obesity, without changes in body weights or islet mass.
Keyword
Figure
Reference
-
1. Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000; 25:406–9.2. Kefalas G, Larose L. PERK leads a hub dictating pancreatic β cell homoeostasis. Biol Cell. 2018; 110:27–32.3. Kim MJ, Min SH, Shin SY, Kim MN, Lee H, Jang JY, et al. Attenuation of PERK enhances glucose-stimulated insulin secretion in islets. J Endocrinol. 2018; 236:125–36.
Article4. Kim MJ, Kim MN, Min SH, Ham DS, Kim JW, Yoon KH, et al. Specific PERK inhibitors enhanced glucose-stimulated insulin secretion in a mouse model of type 2 diabetes. Metabolism. 2019; 97:87–91.
Article5. Rojas-Rivera D, Delvaeye T, Roelandt R, Nerinckx W, Augustyns K, Vandenabeele P, et al. When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ. 2017; 24:1100–10.
Article6. Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001; 7:1153–63.
Article7. Hull RL, Kodama K, Utzschneider KM, Carr DB, Prigeon RL, Kahn SE. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia. 2005; 48:1350–8.
Article8. Wang R, Munoz EE, Zhu S, McGrath BC, Cavener DR. Perk gene dosage regulates glucose homeostasis by modulating pancreatic β-cell functions. PLoS One. 2014; 9:e99684.
Article9. Gupta S, McGrath B, Cavener DR. PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes. 2010; 59:1937–47.10. Sachdeva MM, Stoffers DA. Minireview: meeting the demand for insulin: molecular mechanisms of adaptive postnatal beta-cell mass expansion. Mol Endocrinol. 2009; 23:747–58.11. Ahren J, Ahren B, Wierup N. Increased β-cell volume in mice fed a high-fat diet: a dynamic study over 12 months. Islets. 2010; 2:353–6.
Article12. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004; 306:457–61.
Article13. Zhang W, Hietakangas V, Wee S, Lim SC, Gunaratne J, Cohen SM. ER stress potentiates insulin resistance through PERK-mediated FOXO phosphorylation. Genes Dev. 2013; 27:441–9.
Article14. Fang Z, Gao W, Jiang Q, Loor JJ, Zhao C, Du X, et al. Targeting IRE1α and PERK in the endoplasmic reticulum stress pathway attenuates fatty acid-induced insulin resistance in bovine hepatocytes. J Dairy Sci. 2022; 105:6895–908.
Article15. Nagy C, Einwallner E. Study of in vivo glucose metabolism in high-fat diet-fed mice using oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). J Vis Exp. 2018; 131:56672.16. Lau H, Li S, Corrales N, Rodriguez S, Mohammadi M, Alexander M, et al. Necrostatin-1 supplementation to islet tissue culture enhances the in-vitro development and graft function of young porcine islets. Int J Mol Sci. 2021; 22:8367.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early high-fat feeding improves histone modifications of skeletal muscle at middle-age in mice
- Effects of the Combination of Evogliptin and Leucine on Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice
- A Pilot Study on the Effect of Cannabis Extract on Weight Loss in Diet-induced Obese Mice
- Role of insulin-like growth factor binding protein-3 in glucose and lipid metabolism
- Myeloid-specific SIRT1 Deletion Aggravates Hepatic Inflammation and Steatosis in High-fat Diet-fed Mice