Int J Thyroidol.
2023 Nov;16(2):166-174. 10.11106/ijt.2023.16.2.166.
Comparison of Thyroid-Stimulating Hormone Results from Eight Different Reagents and Assay-Specific Korean Reference Interval for Subclinical Hypothyroidism Treatment
- Affiliations
-
- 1Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea
- 2Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Departments of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 4Departments of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea
- 5Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 6Center for Thyroid Cancer, National Cancer Center, Goyang, Korea
- 7Department of Laboratory Medicine, Eunpyeong St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- 8Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 9Department of Nuclear Medicine, Seoul National University Hospital, Seoul, Korea
- 10Department of Laboratory Medicine, Kangdong Sacred Heart Hospital, Seoul, Korea
- 11Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea
- KMID: 2548744
- DOI: http://doi.org/10.11106/ijt.2023.16.2.166
Abstract
- Background and Objectives
Recent guidelines from the Korean Thyroid Association have proposed a threshold of 6.8 mIU/L for diagnosing subclinical hypothyroidism based on local research findings. However, due to the lack of standardization/harmonization, thyroid-stimulating hormone (TSH) testing yields varying results across different reagent manufacturers. Hence, the use of uniform reference intervals is challenging. We aimed to establish assay-specific Korean reference interval for TSH.
Materials and Methods
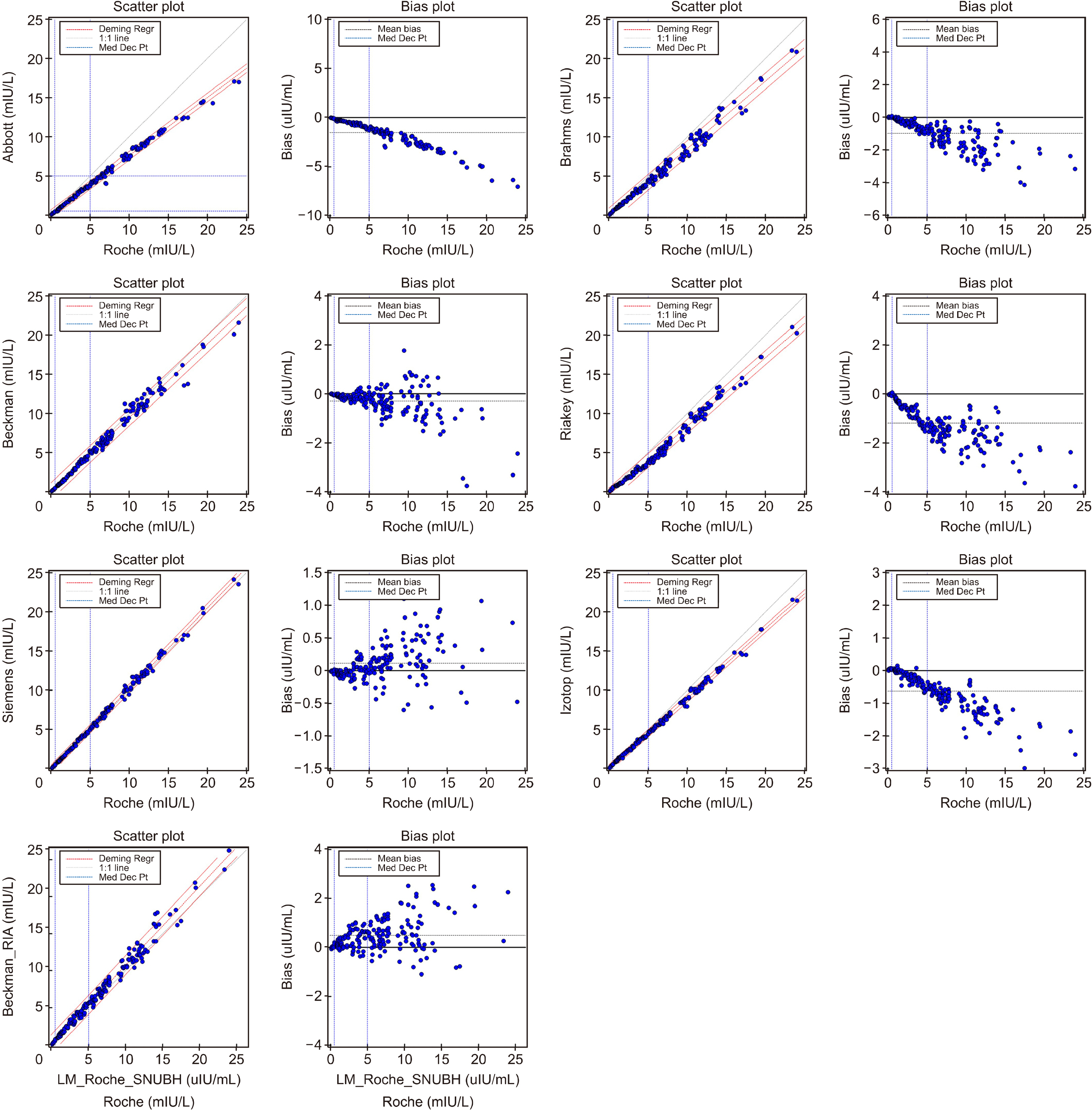
We performed duplicate measurements on 100 serum samples with varying TSH concentrations (0-23 mIU/L) using eight different TSH reagents including Alinity I TSH (Abbott), Access TSH (Beckman Coulter), Elecsys TSH (Roche), TSH3UL (Siemens),TSH IRMA (Beckman Coulter), TSH1 RIA (Brahms), TSH IRMA TUBE II (Riakey), Turbo TSH IRMA (Izotop). Correlation and simple linear regression analyses were conducted among 8 reagents with Roche as the reference.
Results
The correlation coefficient for each reagent was notably high at 0.99. Through regression analysis, TSH values equivalent to the 6.8 mIU/L (Roche) were determined for each reagent as follows: Abbott 5.2 mIU/L, Beckman 6.5 mIU/L, Siemens 6.9 mIU/L, Beckman-Radioimmunoassay 7.4 mIU/L, Brahms 5.7 mIU/L, Riakey 5.3 mIU/L, Izotop 6.0 mIU/L. Conclusion: Given the observed differences in TSH values associated with different reagents, it is imperative to consider these differences when interpreting results within various clinical contexts and adapting them to clinical practice.
Keyword
Figure
Reference
-
References
1. Braverman LE, Kopp PA. 2021. Werner & Ingbar's the thyroid. Wolters Kluwer;Philadelphia, PA, USA:2. Chung HK, Ku EJ, Yoo WS, Kang YE, Kim KJ, Kim BH, et al. 2023; 2023 Korean Thyroid Association management guidelines for patients with subclinical hypothyroidism. Int J Thyroidol. 16(1):32–50. DOI: 10.11106/ijt.2023.16.1.32.
Article3. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. 2014; Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 24(12):1670–751. DOI: 10.1089/thy.2014.0028. PMID: 25266247. PMCID: PMC4267409.
Article4. Pearce SH, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S, et al. 2013; 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2(4):215–28. DOI: 10.1159/000356507. PMID: 24783053. PMCID: PMC3923601.
Article5. Asvold BO, Bjoro T, Vatten LJ. 2009; Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab. 94(12):5023–7. DOI: 10.1210/jc.2009-1180. PMID: 19846737.
Article6. Joung JY, Cho YY, Park SM, Kim TH, Kim NK, Sohn SY, et al. 2014; Effect of iodine restriction on thyroid function in subclinical hypothyroid patients in an iodine-replete area: a long period observation in a large-scale cohort. Thyroid. 24(9):1361–8. DOI: 10.1089/thy.2014.0046. PMID: 24892764.
Article7. Zou Y, Wang D, Cheng X, Ma C, Lin S, Hu Y, et al. 2021; Reference intervals for thyroid-associated hormones and the prevalence of thyroid diseases in the Chinese population. Ann Lab Med. 41(1):77–85. DOI: 10.3343/alm.2021.41.1.77. PMCID: PMC7443523. PMID: 32829582.
Article8. Kim WG, Kim WB, Woo G, Kim H, Cho Y, Kim TY, et al. 2017; Thyroid stimulating hormone reference range and prevalence of thyroid dysfunction in the Korean population: Korea National Health and Nutrition Examination Survey 2013 to 2015. Endocrinol Metab (Seoul). 32(1):106–14. DOI: 10.3803/EnM.2017.32.1.106. PMID: 28116874. PMCID: PMC5368108.
Article9. Jeon MJ, Kim WG, Kwon H, Kim M, Park S, Oh HS, et al. 2017; Excessive iodine intake and thyrotropin reference interval: data from the Korean National Health and Nutrition Examination Survey. Thyroid. 27(7):967–72. DOI: 10.1089/thy.2017.0078. PMID: 28471294.
Article10. Park SY, Kim HI, Oh HK, Kim TH, Jang HW, Chung JH, et al. 2018; Age- and gender-specific reference intervals of TSH and free T4 in an iodine-replete area: data from Korean National Health and Nutrition Examination Survey IV (2013-2015). PLoS One. 13(2):e0190738. DOI: 10.1371/journal.pone.0190738. PMID: 29390008. PMCID: PMC5794073.
Article11. Padoan A, Clerico A, Zaninotto M, Trenti T, Tozzoli R, Aloe R, et al. 2020; Percentile transformation and recalibration functions allow harmonization of thyroid-stimulating hormone (TSH) immunoassay results. Clin Chem Lab Med. 58(10):1663–72. DOI: 10.1515/cclm-2019-1167. PMID: 31927515.
Article12. Thienpont LM, Van Uytfanghe K, De Grande LAC, Reynders D, Das B, Faix JD, et al. 2017; Harmonization of serum thyroid-stimulating hormone measurements paves the way for the adoption of a more uniform reference interval. Clin Chem. 63(7):1248–60. DOI: 10.1373/clinchem.2016.269456. PMID: 28522444.
Article13. Vesper HW, Van Uytfanghe K, Hishinuma A, Raverot V, Patru MM, Danilenko U, et al. 2021; Implementing reference systems for thyroid function tests - a collaborative effort. Clin Chim Acta. 519:183–6. DOI: 10.1016/j.cca.2021.04.019. PMID: 33933427.
Article14. Chung HK, Ku EJ, Yoo WS, Kang YE, Kim KJ, Kim BH, et al. 2023; Erratum: 2023 Korean thyroid association management guidelines for patients with subclinical hypothyroidism. Int J Thyroidol. 16(2):214–5. DOI: 10.11106/ijt.2023.16.2.214.
Article15. CLSI guideline EP28-ED3. Defining, establishing, and verifying reference intervals in the clinical laboratory, 3rd Edition. https://clsi.org/standards/products/method-evaluation/documents/ep28/. cited Nov 14, 2023.16. Adeli K, Higgins V, Trajcevski K, White-Al Habeeb N. 2017; The Canadian laboratory initiative on pediatric reference intervals: a CALIPER white paper. Crit Rev Clin Lab Sci. 54(6):358–413. DOI: 10.1080/10408363.2017.1379945. PMID: 29017389.
Article17. Estey MP, Cohen AH, Colantonio DA, Chan MK, Marvasti TB, Randell E, et al. CLSI-based transference of the CALIPER database of pediatric reference intervals from Abbott to Beckman, Ortho, Roche and Siemens Clinical Chemistry Assays: direct validation using reference samples from the CALIPER cohort. Clin Biochem. 2013; 46(13-14):1197–219. DOI: 10.1016/j.clinbiochem.2013.04.001. PMID: 23578738.
Article18. Higgins V, Chan MK, Nieuwesteeg M, Hoffman BR, Bromberg IL, Gornall D, et al. Transference of CALIPER pediatric reference intervals to biochemical assays on the Roche cobas 6000 and the Roche Modular P. Clin Biochem. 2016; 49(1-2):139–49. DOI: 10.1016/j.clinbiochem.2015.08.018. PMID: 26297116.19. Mu R, Yun K, Yu X, Cheng S, Ma M, Zhang X, et al. 2019; A study on reference interval transference via linear regression. Clin Chem Lab Med. 58(1):116–29. DOI: 10.1515/cclm-2019-0055. PMID: 31352428.20. Kalaria TR, Sanders A, Ford C, Buch H, Fenn JS, Ashby HL, et al. 2022; Biochemical assessment of adequate levothyroxine replacement in primary hypothyroidism differs with different TSH assays: potential clinical implications. J Clin Pathol. 75(6):379–82. DOI: 10.1136/jclinpath-2020-207316. PMID: 33990368.21. Ursem SR, Boelen A, Hillebrand JJ, den Elzen WPJ, Heijboer AC. 2023; How low can we (reliably) go? A method comparison of thyroid-stimulating hormone assays with a focus on low concentrations. Eur Thyroid J. 12(5):e230123. DOI: 10.1530/ETJ-23-0123. PMID: 37552779. PMCID: PMC10503215.
Article22. Kalaria T, Fenn J, Sanders A, Ford C, Gama R. 2022; Clinical concordance assessment should be an integral component of laboratory method comparison studies: a regression transference of routine clinical data approach. Clin Biochem. 103:25–8. DOI: 10.1016/j.clinbiochem.2022.02.008. PMID: 35183526.
Article23. Kalaria T, Sanders A, Fenn J, Ashby HL, Mohammed P, Buch HN, et al. 2021; The diagnosis and management of subclinical hypothyroidism is assay-dependent-implications for clinical practice. Clin Endocrinol (Oxf). 94(6):1012–6. DOI: 10.1111/cen.14423. PMID: 33475154.24. Yamada S, Horiguchi K, Akuzawa M, Sakamaki K, Yamada E, Ozawa A, et al. 2023; The impact of age- and sex-specific reference ranges for serum thyrotropin and free thyroxine on the diagnosis of subclinical thyroid dysfunction: a multicenter study from Japan. Thyroid. 33(4):428–39. DOI: 10.1089/thy.2022.0567. PMID: 36772798. PMCID: PMC10620437.
Article25. Razvi S, Jabbar A, Addison C, Vernazza J, Syed A, Soran H, et al. 2023; Variation in the reference range limits of thyroid function tests and association with the prevalence of levothyroxine treatment. Eur J Endocrinol. 188(2):lvad016. DOI: 10.1093/ejendo/lvad016. PMID: 36751726.
Article26. Clerico A, Ripoli A, Fortunato A, Alfano A, Carrozza C, Correale M, et al. 2017; Harmonization protocols for TSH immunoassays: a multicenter study in Italy. Clin Chem Lab Med. 55(11):1722–33. DOI: 10.1515/cclm-2016-0899. PMID: 28245185.
Article27. Thienpont LM, Faix JD, Beastall G. 2015; Standardization of FT4 and harmonization of TSH measurements - a request for input from endocrinologists and other physicians. Endocr J. 62(10):855–6. DOI: 10.1507/endocrj.EJ15-0382. PMID: 26211473.
Article28. Wheeler E, Choy KW, Chin LK, Wijeratne N, McNeil A, Yen T, et al. 2019; Routine free thyroxine reference intervals are suboptimal for monitoring children on thyroxine replacement therapy and target intervals need to be assay-specific. Sci Rep. 9(1):19080. DOI: 10.1038/s41598-019-55690-x. PMID: 31836869. PMCID: PMC6910984.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Subclinical Thyroid Dysfunction in the Elderly
- A Case of Hypersomnolence with Subclinical Hypothyroidism Treated with Levothyroxine
- Subclinical hypothyroidism in children: updates for pediatricians
- Prevalence and Risk Factors of Subclinical Thyroid Disease
- Clinical Implications of Different Thyroid-Stimulating Hormone (TSH) Reference Intervals between TSH Kits for the Management of Subclinical Hypothyroidism