Neonatal Med.
2023 Nov;30(4):102-107. 10.5385/nm.2023.30.4.102.
Beta Thalassemia Presenting with Neonatal Cholestasis and Extensive Hemosiderosis: A Case Report
- Affiliations
-
- 1Department of Pediatrics, Ajou University School of Medicine, Suwon, Korea
- KMID: 2548300
- DOI: http://doi.org/10.5385/nm.2023.30.4.102
Abstract
- Neonatal cholestasis is caused by various forms of liver injury and has a complex etiological background. Among these, cases of severe cholestasis due to primary hemolytic disease are rare. Herein, we report a case in which thalassemia-induced severe hemolysis caused bile duct injury by hemosiderosis, with cholestasis occurring shortly after birth and lasting for >4 months. In addition, complete recovery of liver pathology was observed both biochemically and histologically. Hence, clinicians should consider hemolytic disease as a rare cause of neonatal cholestasis in the differential diagnosis of neonates as well as the advisability of conservative treatment based on case progression.
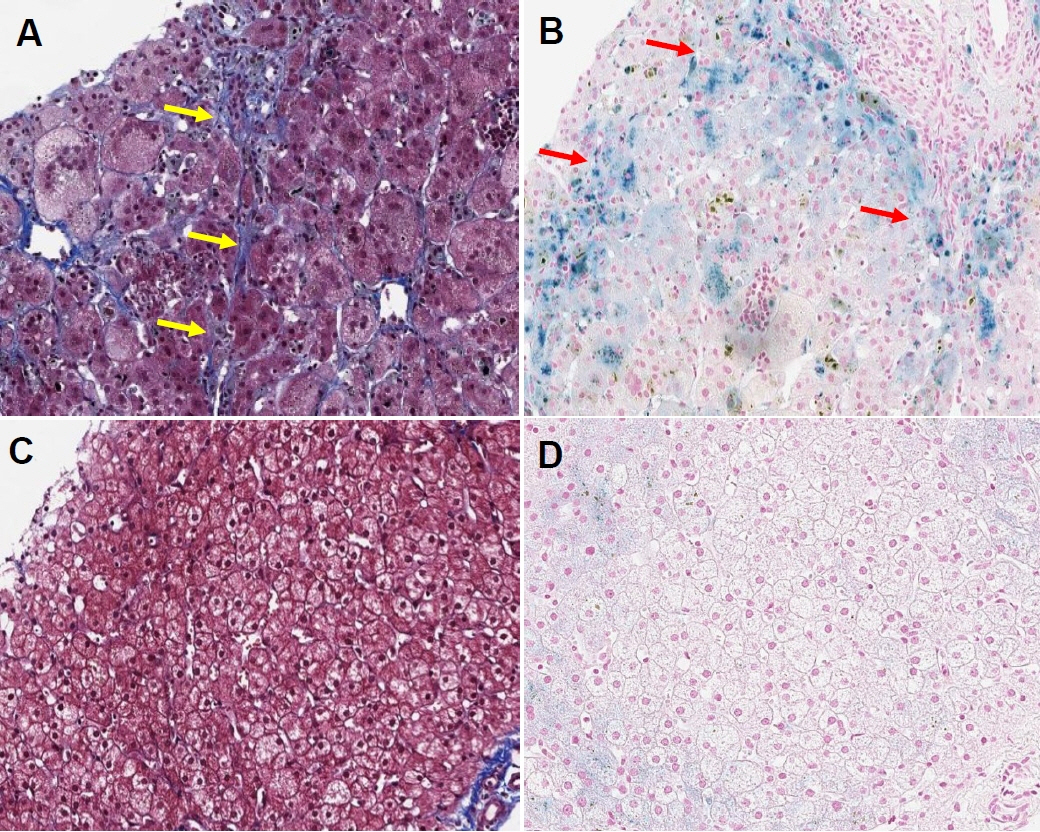
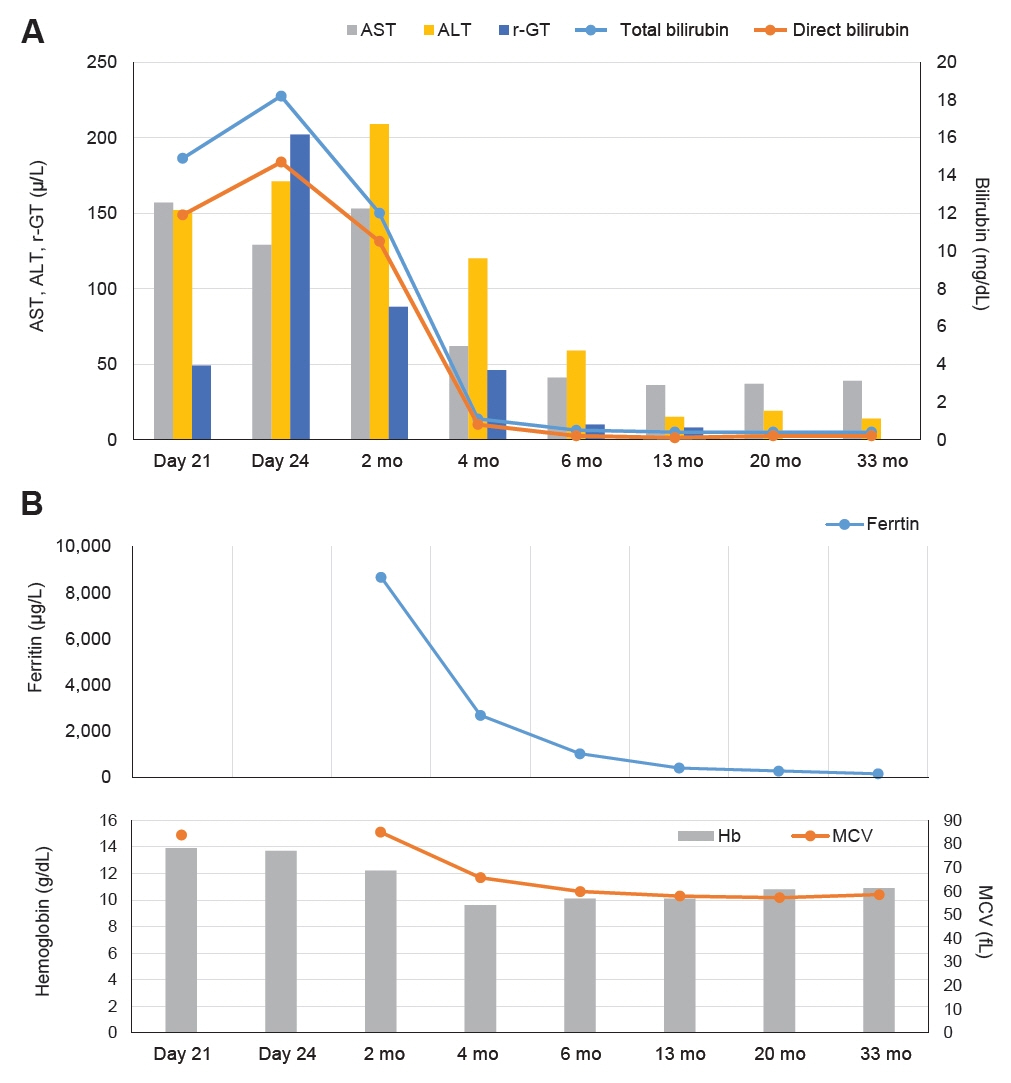
Figure
Reference
-
1. Fawaz R, Baumann U, Ekong U, Fischler B, Hadzic N, Mack CL, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2017; 64:154–68.
Article2. Lane E, Murray KF. Neonatal cholestasis. Pediatr Clin North Am. 2017; 64:621–39.
Article3. Gotze T, Blessing H, Grillhosl C, Gerner P, Hoerning A. Neonatal cholestasis: differential diagnoses, current diagnostic procedures, and treatment. Front Pediatr. 2015; 3:43.4. Gottesman LE, Del Vecchio MT, Aronoff SC. Etiologies of conjugated hyperbilirubinemia in infancy: a systematic review of 1692 subjects. BMC Pediatr. 2015; 15:192.
Article5. Teng J, Wickman L, Reilly M, Nemeth A, Fischler B, Bohlin K, et al. Population-based incidence and risk factors for cholestasis in hemolytic disease of the fetus and newborn. J Perinatol. 2022; 42:702–7.
Article6. Cao A, Galanello R. Beta-thalassemia. Genet Med. 2010; 12:61–76.
Article7. Lee HJ, Shin KH, Kim HH, Yang EJ, Park KH, Kim MJ, et al. Increased prevalence of thalassemia in young people in Korea: impact of increasing immigration. Ann Lab Med. 2019; 39:133–40.
Article8. Lee JS, Rhee TM, Jeon K, Cho Y, Lee SW, Han KD, et al. Epidemiologic trends of thalassemia, 2006-2018: a nationwide population-based study. J Clin Med. 2022; 11:2289.
Article9. Hong CR, Kang HJ, Lee JW, Kim H, Kim NH, Park KD, et al. Clinical characteristics of pediatric thalassemia in Korea: a single institute experience. J Korean Med Sci. 2013; 28:1645–9.
Article10. Bender MA, Hulihan M, Dorley MC, Aguinaga MDP, Ojodu J, Yusuf C. Newborn screening practices for beta-thalassemia in the United States. Int J Neonatal Screen. 2021; 7:83.
Article11. Qin LQ, Yan TZ, Luo SQ, Cai PF, Chen LZ, Zhong QY, et al. Application of DNA microarray in genetic mutation detection in patients with thalassemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021; 29:1561–5.12. Rizvi SA, Ge L, Li S, Pandey A. Acute liver failure in a sickle cell patient. J Pediatr Gastroenterol Nutr. 2021; 72:e54–5.
Article13. Kotch C, Friedman DF, Wilkins BJ, Samelson-Jones BJ. Conservative management of alloimmune hemolysis and cholestasis with extreme laboratory abnormalities. Pediatrics. 2021; 147:e20193367.
Article14. Saeed O, Panarelli N, Umrau K, Lee H, Westerhoff M, Cheng J, et al. The histopathologic features of sickle cell hepatopathy: a multi-institutional study. Am J Clin Pathol. 2022; 157:73–81.
Article15. Silver MM, Valberg LS, Cutz E, Lines LD, Phillips MJ. Hepatic morphology and iron quantitation in perinatal hemochromatosis: comparison with a large perinatal control population, including cases with chronic liver disease. Am J Pathol. 1993; 143:1312–25.16. Smith SR, Shneider BL, Magid M, Martin G, Rothschild M. Minor salivary gland biopsy in neonatal hemochromatosis. Arch Otolaryngol Head Neck Surg. 2004; 130:760–3.
Article17. Chavhan GB, Kamath BM, Siddiqui I, Tomlinson C. Magnetic resonance imaging of neonatal hemochromatosis. Pediatr Radiol. 2022; 52:334–9.
Article18. Kountouras D, Tsagarakis NJ, Fatourou E, Dalagiorgos E, Chrysanthos N, Berdoussi H, et al. Liver disease in adult transfusiondependent beta-thalassaemic patients: investigating the role of iron overload and chronic HCV infection. Liver Int. 2013; 33:420–7.
Article