J Rhinol.
2023 Nov;30(3):155-160. 10.18787/jr.2023.00061.
A Survey on Biologics for the Treatment of Chronic Rhinosinusitis With Nasal Polyps Among Members of the Korean Rhinologic Society
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Republic of Korea
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 3Department of Otorhinolaryngology, Dankook University College of Medicine, Cheonan, Republic of Korea
- 4Beckman Laser Institute Korea, Dankook University College of Medicine, Cheonan, Republic of Korea
- 5Laser Translational Clinical Trial Center, Dankook University Hospital, Cheonan, Republic of Korea
- KMID: 2548193
- DOI: http://doi.org/10.18787/jr.2023.00061
Abstract
- Background and Objectives
In 2021, biologics were approved for treating chronic rhinosinusitis with nasal polyps (CRSwNP) in Korea. However, CRS is a heterogeneous disease, and its characteristics are thought to differ between Western and Korean populations. This study aimed to evaluate the experiences of members of the Korean Rhinologic Society during the first year of biologic usage for the treatment of nasal polyps.
Methods
An anonymous survey consisting of 15 items was conducted from November to December 2021. The survey included questions about participant demographics, use of biologics for treating CRSwNP, and expectations regarding the effectiveness of biologics for treating CRSwNP.
Results
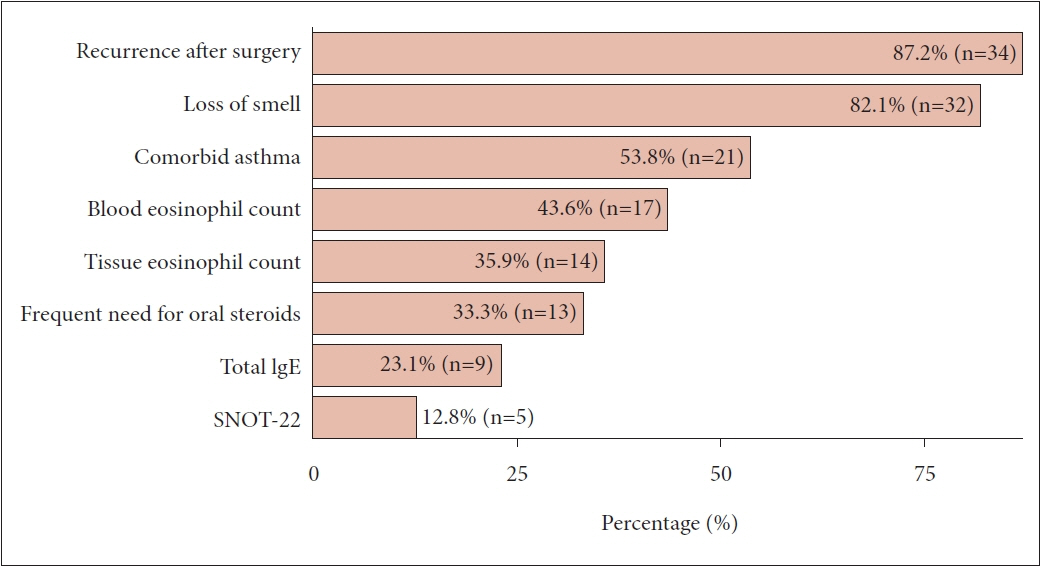
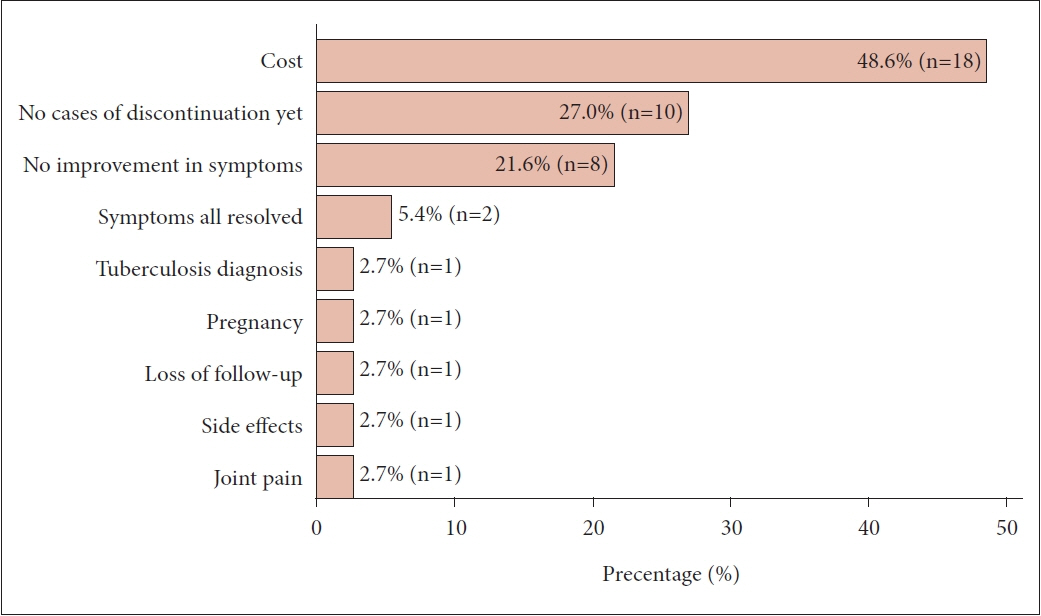
In total, 44 members participated in the survey. Approximately half of the respondents were in their 40s (50.0%) and had 5–9 years of clinical experience as otorhinolaryngologists (47.7%). The majority of participants held academic positions (95.4%). About half of them worked in Gyeonggi Province. The utilization of biologics did not differ significantly based on clinical experience (p=0.192). When asked about the factors considered for prescribing biologics, the most common reason was recurrence of polyps after surgery (87.2%). The most frequent reason for discontinuing biologics was cost (48.6%). When asked about the extent to which they expected that the availability of biologics for CRSwNP treatment would reduce endoscopic sinus surgery (ESS), 45.5% of members expected a reduction of approximately 10%–29%. In addition, 20.5% expected a reduction of 50% or more. However, 61.4% expected a reduction of less than 10% in primary ESS. In addition, most respondents (93.2%) agreed with the need for Korea-specific guidelines for biologic treatment.
Conclusion
There are discrepancies between the current guidelines for biologic treatment of CRSwNP and the reality of the situation, highlighting the need for the development of Korea-specific guidelines.
Keyword
Figure
Reference
-
References
1. Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011; 25(3):117–21.2. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020; 58(Suppl 29):1–464.3. Khalmuratova R, Shin HW. Crosstalk between mucosal inflammation and bone metabolism in chronic rhinosinusitis. Clin Exp Otorhinolaryngol. 2021; 14(1):43–9.
Article4. Ueki S, Tokunaga T, Fujieda S, Honda K, Hirokawa M, Spencer LA, et al. Eosinophil ETosis and DNA traps: a new look at eosinophilic inflammation. Curr Allergy Asthma Rep. 2016; 16(8):54.
Article5. Kim DW, Yang SK. Application of biologics in treating chronic rhinosinusitis with nasal polyps in Asian populations. Clin Exp Otorhinolaryngol. 2022; 15(2):125–6.
Article6. Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016; 137(5):1449–56. e4.
Article7. Wang X, Sima Y, Zhao Y, Zhang N, Zheng M, Du K, et al. Endotypes of chronic rhinosinusitis based on inflammatory and remodeling factors. J Allergy Clin Immunol. 2023; 151(2):458–68.
Article8. Min JY, Kim JY, Sung CM, Kim ST, Cho HJ, Mun SJ, et al. Inflammatory endotypes of chronic rhinosinusitis in the Korean population: distinct expression of type 3 inflammation. Allergy Asthma Immunol Res. 2023; 15(4):437–50.
Article9. Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016; 138(5):1344–53.10. Kim DW. Can neutrophils be a cellular biomarker in Asian chronic rhinosinusitis? Clin Exp Otorhinolaryngol. 2019; 12(4):325–6.
Article11. Gevaert P, Omachi TA, Corren J, Mullol J, Han J, Lee SE, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020; 146(3):595–605.
Article12. Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, doubleblind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019; 394(10209):1638–50.
Article13. Fokkens WJ, Viskens AS, Backer V, Conti D, De Corso E, Gevaert P, et al. EPOS/EUFOREA update on indication and evaluation of biologics in chronic rhinosinusitis with nasal polyps 2023. Rhinology. 2023; 61(3):194–202.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medical treatment according to phenotypes of chronic rhinosinusitis
- Practical Review of Biologics in Chronic Rhinosinusitis With Nasal Polyps
- Update on Biologics in Treatment of Chronic Rhinosinusitis With Nasal Polyposis
- Endotypes of Asian chronic rhinosinusitis with nasal polyps: A narrative review
- Tissue Remodeling in Rhinosinusitis