Diabetes Metab J.
2023 Nov;47(6):818-825. 10.4093/dmj.2023.0171.
The Efficacy and Safety of Moderate-Intensity Rosuvastatin with Ezetimibe versus High-Intensity Rosuvastatin in High Atherosclerotic Cardiovascular Disease Risk Patients with Type 2 Diabetes Mellitus: A Randomized, Multicenter, Open, Parallel, Phase 4 Study
- Affiliations
-
- 1Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea
- 2Department of Internal Medicine and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- 3Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul,
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 6Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- 7Department of Internal Medicine, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 8Clinical Operation Team, Yuhan Corporation, Seoul, Korea
- KMID: 2548158
- DOI: http://doi.org/10.4093/dmj.2023.0171
Abstract
- Background
To investigate the efficacy and safety of moderate-intensity rosuvastatin/ezetimibe combination compared to highintensity rosuvastatin in high atherosclerotic cardiovascular disease (ASCVD) risk patients with type 2 diabetes mellitus (T2DM).
Methods
This study was a randomized, multicenter, open, parallel phase 4 study, and enrolled T2DM subjects with an estimated 10-year ASCVD risk ≥7.5%. The primary endpoint was the low-density lipoprotein cholesterol (LDL-C) change rate after 24-week rosuvastatin 10 mg/ezetimibe 10 mg treatment was non-inferior to that of rosuvastatin 20 mg. The achievement proportion of 10-year ASCVD risk <7.5% or comprehensive lipid target (LDL-C <70 mg/dL, non-high-density lipoprotein cholesterol <100 mg/dL, and apolipoprotein B <80 mg/dL) without discontinuation, and several metabolic parameters were explored as secondary endpoints.
Results
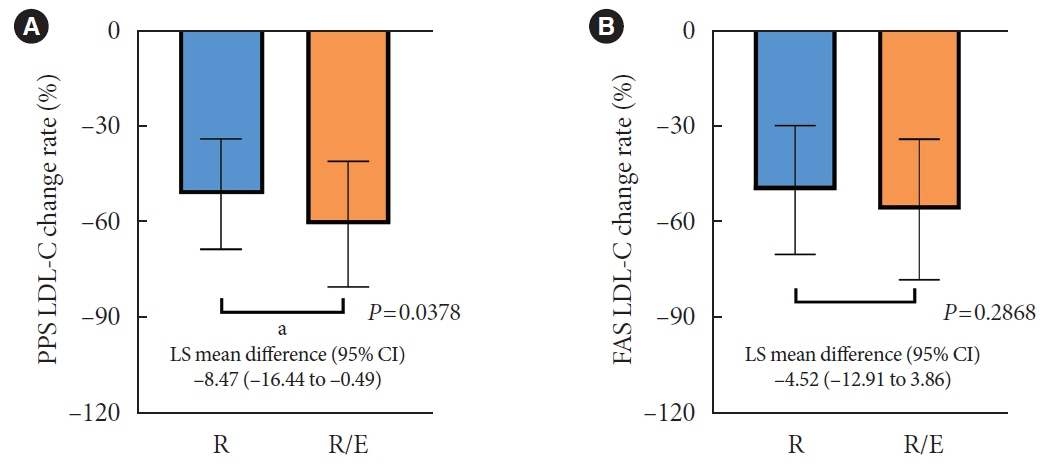
A hundred and six participants were assigned to each group. Both groups showed significant reduction in % change of LDL-C from baseline at week 24 (–63.90±6.89 vs. –55.44±6.85, combination vs. monotherapy, p=0.0378; respectively), but the combination treatment was superior to high-intensity monotherapy in LDL-C change (%) from baseline (least square [LS] mean difference, –8.47; 95% confidence interval, –16.44 to –0.49; p=0.0378). The combination treatment showed a higher proportion of achieved comprehensive lipid targets rather than monotherapy (85.36% vs. 62.22% in monotherapy, p=0.015). The ezetimibe combination significantly improved homeostasis model assessment of β-cell function even without A1c changes (LS mean difference, 17.13; p=0.0185).
Conclusion
In high ASCVD risk patients with T2DM, the combination of moderate-intensity rosuvastatin and ezetimibe was not only non-inferior but also superior to improving dyslipidemia with additional benefits compared to high-intensity rosuvastatin monotherapy.
Keyword
Figure
Cited by 1 articles
-
Does Rosuvastatin/Ezetimibe Combination Therapy Offer Potential Benefits for Glucose Metabolism beyond Lipid-Lowering Efficacy in T2DM?
Il Rae Park, Jun Sung Moon
Diabetes Metab J. 2024;48(3):387-389. doi: 10.4093/dmj.2024.0168.
Reference
-
1. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010; 56:1113–32.2. Tomlinson B, Patil NG, Fok M, Lam CW. Managing dyslipidemia in patients with type 2 diabetes. Expert Opin Pharmacother. 2021; 22:2221–34.
Article3. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014; 63(25 Pt B):2889–934.4. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019; 73:3168–209.5. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007; 99:410–4.
Article6. Mulder AB, van Lijf HJ, Bon MA, van den Bergh FA, Touw DJ, Neef C, et al. Association of polymorphism in the cytochrome CYP2D6 and the efficacy and tolerability of simvastatin. Clin Pharmacol Ther. 2001; 70:546–51.
Article7. Naito R, Miyauchi K, Daida H. Racial differences in the cholesterol-lowering effect of statin. J Atheroscler Thromb. 2017; 24:19–25.
Article8. Gagne C, Bays HE, Weiss SR, Mata P, Quinto K, Melino M, et al. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol. 2002; 90:1084–91.
Article9. Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003; 107:2409–15.
Article10. Kim BK, Hong SJ, Lee YJ, Hong SJ, Yun KH, Hong BK, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022; 400:380–90.
Article11. Hwang YC, Jun JE, Jeong IK, Ahn KJ, Chung HY. Comparison of the efficacy of rosuvastatin monotherapy 20 mg with rosuvastatin 5 mg and ezetimibe 10 mg combination therapy on lipid parameters in patients with type 2 diabetes mellitus. Diabetes Metab J. 2019; 43:582–9.
Article12. Lee J, Hwang YC, Lee WJ, Won JC, Song KH, Park CY, et al. Comparison of the efficacy and safety of rosuvastatin/ezetimibe combination therapy and rosuvastatin monotherapy on lipoprotein in patients with type 2 diabetes: multicenter randomized controlled study. Diabetes Ther. 2020; 11:859–71.
Article13. Rhee MY, Kim KJ, Kim SH, Yoon YW, Rha SW, Hong SJ, et al. Ezetimibe and rosuvastatin combination treatment can reduce the dose of rosuvastatin without compromising its lipid-lowering efficacy. Clin Ther. 2019; 41:2571–92.
Article14. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012; 380:565–71.
Article15. Kim YS, Han YE, Choi EA, You NY, Lee JW, You HS, et al. Statin use increased new-onset diabetes in hypercholesterolemic individuals: data from the Korean National Health Insurance Service-National Health Screening Cohort database (NHIS-HEALS). Prim Care Diabetes. 2020; 14:246–53.
Article16. Carmena R, Betteridge DJ. Diabetogenic action of statins: mechanisms. Curr Atheroscler Rep. 2019; 21:23.
Article17. Abbasi F, Lamendola C, Harris CS, Harris V, Tsai MS, Tripathi P, et al. Statins are associated with increased insulin resistance and secretion. Arterioscler Thromb Vasc Biol. 2021; 41:2786–97.
Article18. Yang SJ, Choi JM, Kim L, Kim BJ, Sohn JH, Kim WJ, et al. Chronic administration of ezetimibe increases active glucagonlike peptide-1 and improves glycemic control and pancreatic beta cell mass in a rat model of type 2 diabetes. Biochem Biophys Res Commun. 2011; 407:153–7.
Article19. Yoon JS, Moon JS, Kim YW, Won KC, Lee HW. The glucotoxicity protecting effect of ezetimibe in pancreatic beta cells via inhibition of CD36. J Korean Med Sci. 2016; 31:547–52.
Article20. Riaz H, Khan AR, Khan MS, Rehman KA, Alansari SA, Gheyath B, et al. Meta-analysis of placebo-controlled randomized controlled trials on the prevalence of statin intolerance. Am J Cardiol. 2017; 120:774–81.
Article21. Thakker D, Nair S, Pagada A, Jamdade V, Malik A. Statin use and the risk of developing diabetes: a network meta-analysis. Pharmacoepidemiol Drug Saf. 2016; 25:1131–49.
Article22. Thongtang N, Tangkittikasem N, Samaithongcharoen K, Piyapromdee J, Srinonprasert V, Sriussadaporn S. Effect of switching from low-dose simvastatin to high-dose atorvastatin on glucose homeostasis and cognitive function in type 2 diabetes. Vasc Health Risk Manag. 2020; 16:367–77.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lipid-Lowering Efficacy of Combination Therapy With ModerateIntensity Statin and Ezetimibe Versus High-Intensity Statin Monotherapy: A Randomized, Open-Label, NonInferiority Trial From Korea
- Letter: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J 2019;43:582–9)
- Response: Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J 2019;43:582–9)
- Comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus
- Moderate-Intensity Rosuvastatin/Ezetimibe Combination versus Quadruple-Dose Rosuvastatin Monotherapy: A Meta-Analysis and Systemic Review