Diabetes Metab J.
2023 Nov;47(6):743-756. 10.4093/dmj.2023.0018.
Pharmacological and Nonpharmacological Treatments for Painful Diabetic Peripheral Neuropathy
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2548150
- DOI: http://doi.org/10.4093/dmj.2023.0018
Abstract
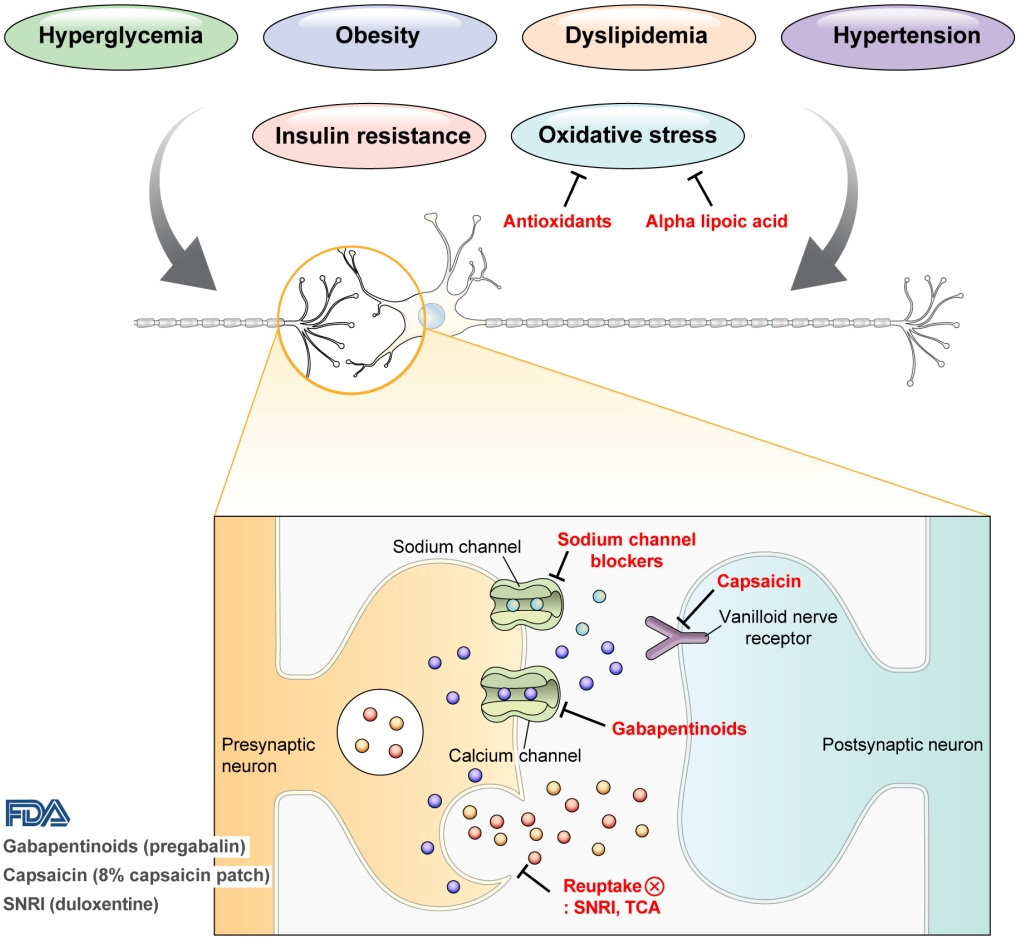
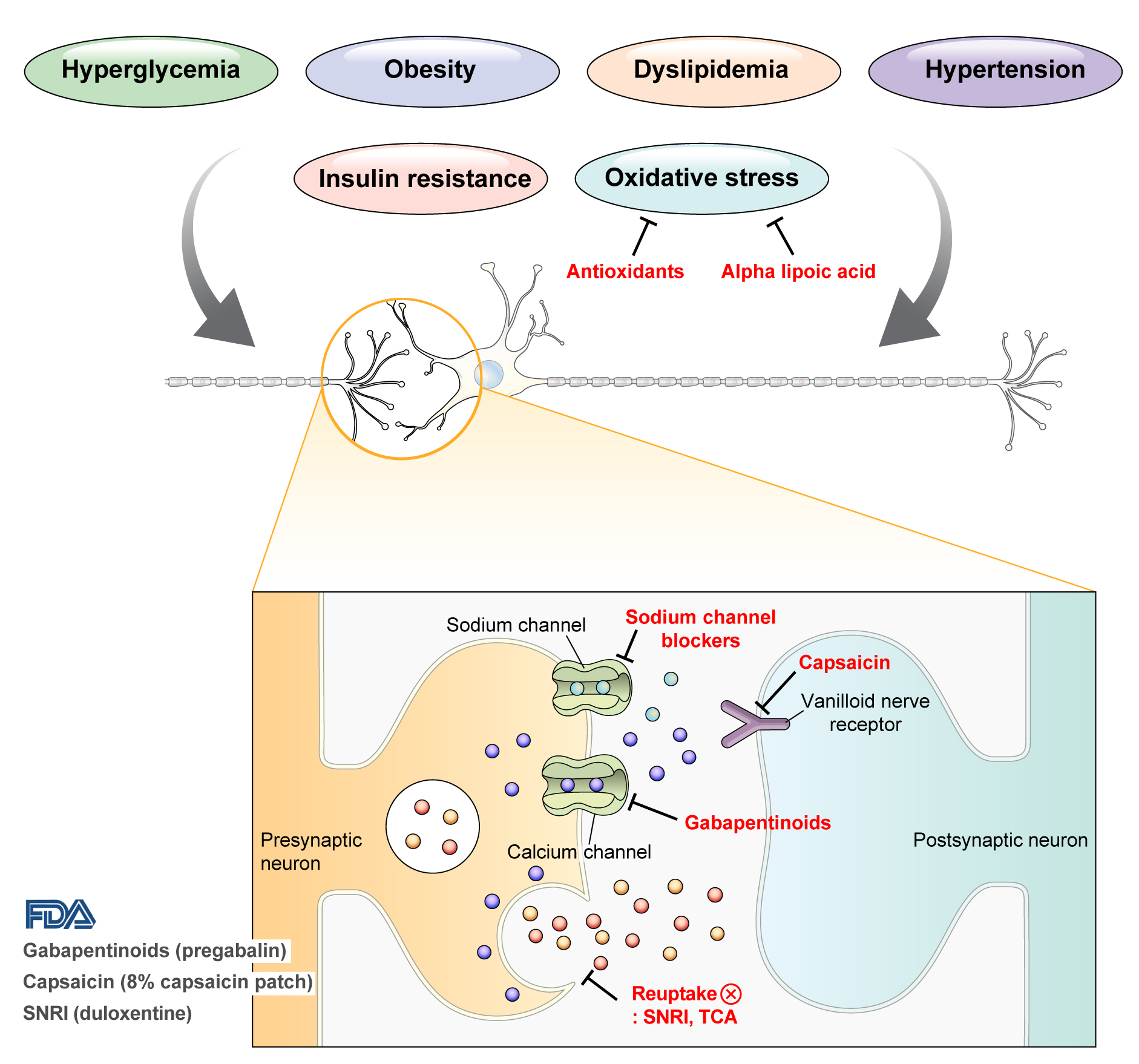
- Diabetic peripheral neuropathy (DPN) is one of the most prevalent chronic complications of diabetes. The lifetime prevalence of DPN is thought to be >50%, and 15%–25% of patients with diabetes experience neuropathic pain, referred to as “painful DPN.” Appropriate treatment of painful DPN is important because this pain contributes to a poor quality of life by causing sleep disturbance, anxiety, and depression. The basic principle for the management of painful DPN is to control hyperglycemia and other modifiable risk factors, but these may be insufficient for preventing or improving DPN. Because there is no promising diseasemodifying medication for DPN, the pain itself needs to be managed when treating painful DPN. Drugs for neuropathic pain, such as gabapentinoids, serotonin–norepinephrine reuptake inhibitors, tricyclic antidepressants, alpha-lipoic acid, sodium channel blockers, and topical capsaicin, are used for the management of painful DPN. The U.S. Food and Drug Administration (FDA) has approved pregabalin, duloxetine, tapentadol, and the 8% capsaicin patch as drugs for the treatment of painful DPN. Recently, spinal cord stimulation using electrical stimulation is approved by the FDA for the treatment for painful DPN. This review describes the currently available pharmacological and nonpharmacological treatments for painful DPN.
Keyword
Figure
Reference
-
1. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017; 40:136–54.
Article2. Shillo P, Sloan G, Greig M, Hunt L, Selvarajah D, Elliott J, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep. 2019; 19:32.
Article3. Ziegler D, Landgraf R, Lobmann R, Reiners K, Rett K, Schnell O, et al. Painful and painless neuropathies are distinct and largely undiagnosed entities in subjects participating in an educational initiative (PROTECT study). Diabetes Res Clin Pract. 2018; 139:147–54.
Article4. Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017; 3:17002.
Article5. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414:813–20.
Article6. Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010; 376:419–30.
Article7. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360:129–39.
Article8. Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014; 14:528.
Article9. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012; 11:521–34.
Article10. Callaghan BC, Xia R, Reynolds E, Banerjee M, Rothberg AE, Burant CF, et al. Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol. 2016; 73:1468–76.
Article11. Oh TJ, Lee JE, Choi SH, Jang HC. Association between body fat and diabetic peripheral neuropathy in middle-aged adults with type 2 diabetes mellitus: a preliminary report. J Obes Metab Syndr. 2019; 28:112–7.
Article12. Elafros MA, Andersen H, Bennett DL, Savelieff MG, Viswanathan V, Callaghan BC, et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022; 21:922–36.
Article13. Kim K, Oh TJ, Park YS, Chang W, Cho HC, Lee J, et al. Association between fat mass or fat fibrotic gene expression and polyneuropathy in subjects with obesity: a Korean Metabolic Bariatric Surgery Cohort. Front Endocrinol (Lausanne). 2022; 13:881093.
Article14. Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig. 2017; 8:646–55.
Article15. Bonhof GJ, Strom A, Puttgen S, Ringel B, Bruggemann J, Bodis K, et al. Patterns of cutaneous nerve fibre loss and regeneration in type 2 diabetes with painful and painless polyneuropathy. Diabetologia. 2017; 60:2495–503.
Article16. Look AHEAD Research Group. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia. 2017; 60:980–8.17. Singh P, Adderley N, Subramanian A, Gokhale K, Singhal R, Toulis KA, et al. The impact of bariatric surgery on incident microvascular complications in patients with type 2 diabetes: a matched controlled population-based retrospective cohort study. Diabetes Care. 2021; 44:116–24.
Article18. Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006; 20:216–23.
Article19. Singleton JR, Marcus RL, Jackson JE, Lessard MK, Graham TE, Smith AG. Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Ann Clin Transl Neurol. 2014; 1:844–9.
Article20. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020; 161:1976–82.
Article21. Yang M, Qian C, Liu Y. Suboptimal treatment of diabetic peripheral neuropathic pain in the United States. Pain Med. 2015; 16:2075–83.
Article22. Moon SS, Kim CH, Kang SM, Kim ES, Oh TJ, Yun JS, et al. Status of diabetic neuropathy in Korea: a National Health Insurance Service-National Sample Cohort analysis (2006 to 2015). Diabetes Metab J. 2021; 45:115–9.
Article23. Chincholkar M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. Br J Anaesth. 2018; 120:1315–34.
Article24. Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998; 280:1831–6.
Article25. Mathieson S, Lin CC, Underwood M, Eldabe S. Pregabalin and gabapentin for pain. BMJ. 2020; 369:m1315.
Article26. Sandercock D, Cramer M, Wu J, Chiang YK, Biton V, Heritier M. Gabapentin extended release for the treatment of painful diabetic peripheral neuropathy: efficacy and tolerability in a double-blind, randomized, controlled clinical trial. Diabetes Care. 2009; 32:e20.27. Dallocchio C, Buffa C, Mazzarello P, Chiroli S. Gabapentin vs. amitriptyline in painful diabetic neuropathy: an open-label pilot study. J Pain Symptom Manage. 2000; 20:280–5.28. McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacol. 2004; 4:14.29. Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004; 110:628–38.
Article30. Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004; 63:2104–10.
Article31. Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005; 6:253–60.
Article32. Huffman C, Stacey BR, Tuchman M, Burbridge C, Li C, Parsons B, et al. Efficacy and safety of pregabalin in the treatment of patients with painful diabetic peripheral neuropathy and pain on walking. Clin J Pain. 2015; 31:946–58.
Article33. Mu Y, Liu X, Li Q, Chen K, Liu Y, Lv X, et al. Efficacy and safety of pregabalin for painful diabetic peripheral neuropathy in a population of Chinese patients: a randomized placebo-controlled trial. J Diabetes. 2018; 10:256–65.
Article34. Parsons B, Li C. The efficacy of pregabalin in patients with moderate and severe pain due to diabetic peripheral neuropathy. Curr Med Res Opin. 2016; 32:929–37.
Article35. Hurley RW, Lesley MR, Adams MC, Brummett CM, Wu CL. Pregabalin as a treatment for painful diabetic peripheral neuropathy: a meta-analysis. Reg Anesth Pain Med. 2008; 33:389–94.
Article36. Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008; 31:1448–54.37. Vinik A, Rosenstock J, Sharma U, Feins K, Hsu C, Merante D, et al. Efficacy and safety of mirogabalin (DS-5565) for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo- and active comparator-controlled, adaptive proof-of-concept phase 2 study. Diabetes Care. 2014; 37:3253–61.
Article38. Merante D, Rosenstock J, Sharma U, Feins K, Hsu C, Vinik A, et al. Efficacy of mirogabalin (DS-5565) on patient-reported pain and sleep interference in patients with diabetic neuropathic pain: secondary outcomes of a phase II proof-of-concept study. Pain Med. 2017; 18:2198–207.
Article39. Baba M, Matsui N, Kuroha M, Wasaki Y, Ohwada S. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase III study in Asian patients. J Diabetes Investig. 2019; 10:1299–306.40. Mellegers MA, Furlan AD, Mailis A. Gabapentin for neuropathic pain: systematic review of controlled and uncontrolled literature. Clin J Pain. 2001; 17:284–95.
Article41. Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010; 49:661–9.
Article42. Raouf M, Atkinson TJ, Crumb MW, Fudin J. Rational dosing of gabapentin and pregabalin in chronic kidney disease. J Pain Res. 2017; 10:275–8.
Article43. Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, et al. A comprehensive algorithm for management of neuropathic pain. Pain Med. 2019; 20(Suppl 1):S2–12.
Article44. Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. Efficacy of duloxetine, a potent and balanced serotoninnorepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004; 311:576–84.
Article45. Detke MJ, Lu Y, Goldstein DJ, Hayes JR, Demitrack MA. Duloxetine, 60 mg once daily, for major depressive disorder: a randomized double-blind placebo-controlled trial. J Clin Psychiatry. 2002; 63:308–15.46. Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005; 116:109–18.
Article47. Rudroju N, Bansal D, Talakokkula ST, Gudala K, Hota D, Bhansali A, et al. Comparative efficacy and safety of six antidepressants and anticonvulsants in painful diabetic neuropathy: a network meta-analysis. Pain Physician. 2013; 16:E705–14.48. Griebeler ML, Morey-Vargas OL, Brito JP, Tsapas A, Wang Z, Carranza Leon BG, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. 2014; 161:639–49.
Article49. Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004; 110:697–706.
Article50. Sindrup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003; 60:1284–9.
Article51. Razazian N, Baziyar M, Moradian N, Afshari D, Bostani A, Mahmoodi M. Evaluation of the efficacy and safety of pregabalin, venlafaxine, and carbamazepine in patients with painful diabetic peripheral neuropathy: a randomized, double-blind trial. Neurosciences (Riyadh). 2014; 19:192–8.52. Boulton AJ. Management of diabetic peripheral neuropathy. Clin Diabetes. 2005; 23:9–15.
Article53. Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992; 326:1250–6.
Article54. Ulugol A, Karadag HC, Tamer M, Firat Z, Aslantas A, Dokmeci I. Involvement of adenosine in the anti-allodynic effect of amitriptyline in streptozotocin-induced diabetic rats. Neurosci Lett. 2002; 328:129–32.
Article55. Khdour MR. Treatment of diabetic peripheral neuropathy: a review. J Pharm Pharmacol. 2020; 72:863–72.
Article56. Max MB, Culnane M, Schafer SC, Gracely RH, Walther DJ, Smoller B, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987; 37:589–96.
Article57. Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med. 1999; 159:1931–7.
Article58. Bansal D, Bhansali A, Hota D, Chakrabarti A, Dutta P. Amitriptyline vs. pregabalin in painful diabetic neuropathy: a randomized double blind clinical trial. Diabet Med. 2009; 26:1019–26.
Article59. Kaur H, Hota D, Bhansali A, Dutta P, Bansal D, Chakrabarti A. A comparative evaluation of amitriptyline and duloxetine in painful diabetic neuropathy: a randomized, double-blind, cross-over clinical trial. Diabetes Care. 2011; 34:818–22.60. Benbouzid M, Gaveriaux-Ruff C, Yalcin I, Waltisperger E, Tessier LH, Muller A, et al. Delta-opioid receptors are critical for tricyclic antidepressant treatment of neuropathic allodynia. Biol Psychiatry. 2008; 63:633–6.
Article61. Low PA, Nickander KK, Tritschler HJ. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes. 1997; 46 Suppl 2:S38–42.
Article62. Nagamatsu M, Nickander KK, Schmelzer JD, Raya A, Wittrock DA, Tritschler H, et al. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care. 1995; 18:1160–7.
Article63. Nickander KK, McPhee BR, Low PA, Tritschler H. Alpha-lipoic acid: antioxidant potency against lipid peroxidation of neural tissues in vitro and implications for diabetic neuropathy. Free Radic Biol Med. 1996; 21:631–9.
Article64. Garrett NE, Malcangio M, Dewhurst M, Tomlinson DR. alpha-Lipoic acid corrects neuropeptide deficits in diabetic rats via induction of trophic support. Neurosci Lett. 1997; 222:191–4.65. Hounsom L, Horrobin DF, Tritschler H, Corder R, Tomlinson DR. A lipoic acid-gamma linolenic acid conjugate is effective against multiple indices of experimental diabetic neuropathy. Diabetologia. 1998; 41:839–43.
Article66. Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schutte K, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid: a 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia. 1995; 38:1425–33.
Article67. Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schutte K, et al. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care. 1999; 22:1296–301.
Article68. Spiller HA, Carlisle RD. Status epilepticus after massive carbamazepine overdose. J Toxicol Clin Toxicol. 2002; 40:81–90.
Article69. Dogra S, Beydoun S, Mazzola J, Hopwood M, Wan Y. Oxcarbazepine in painful diabetic neuropathy: a randomized, placebo-controlled study. Eur J Pain. 2005; 9:543–54.
Article70. Erdemoglu AK, Varlibas A. Effectiveness of oxcarbazepine in symptomatic treatment of painful diabetic neuropathy. Neurol India. 2006; 54:173–7.71. Zhou M, Chen N, He L, Yang M, Zhu C, Wu F. Oxcarbazepine for neuropathic pain. Cochrane Database Syst Rev. 2017; 12:CD007963.
Article72. Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1: an update. Eur J Biochem. 2004; 271:1814–9.73. Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% Capsaicin gel for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. Pain Pract. 2013; 13:497–503.
Article74. Kulkantrakorn K, Chomjit A, Sithinamsuwan P, Tharavanij T, Suwankanoknark J, Napunnaphat P. 0.075% Capsaicin lotion for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. J Clin Neurosci. 2019; 62:174–9.
Article75. Simpson DM, Robinson-Papp J, Van J, Stoker M, Jacobs H, Snijder RJ, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebocontrolled study. J Pain. 2017; 18:42–53.
Article76. Abrams RMC, Pedowitz EJ, Simpson DM. A critical review of the capsaicin 8% patch for the treatment of neuropathic pain associated with diabetic peripheral neuropathy of the feet in adults. Expert Rev Neurother. 2021; 21:259–66.
Article77. Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003; 9:294–9.
Article78. Coste T, Pierlovisi M, Leonardi J, Dufayet D, Gerbi A, Lafont H, et al. Beneficial effects of gamma linolenic acid supplementation on nerve conduction velocity, Na+, K+ ATPase activity, and membrane fatty acid composition in sciatic nerve of diabetic rats. J Nutr Biochem. 1999; 10:411–20.
Article79. Satoh J, Kohara N, Sekiguchi K, Yamaguchi Y. Effect of ranirestat on sensory and motor nerve function in Japanese patients with diabetic polyneuropathy: a randomized double-blind placebo-controlled study. J Diabetes Res. 2016; 2016:5383797.
Article80. Sekiguchi K, Kohara N, Baba M, Komori T, Naito Y, Imai T, et al. Aldose reductase inhibitor ranirestat significantly improves nerve conduction velocity in diabetic polyneuropathy: a randomized double-blind placebo-controlled study in Japan. J Diabetes Investig. 2019; 10:466–74.
Article81. Xie J, Strauss VY, Martinez-Laguna D, Carbonell-Abella C, Diez-Perez A, Nogues X, et al. Association of tramadol vs codeine prescription dispensation with mortality and other adverse clinical outcomes. JAMA. 2021; 326:1504–15.
Article82. Price R, Smith D, Franklin G, Gronseth G, Pignone M, David WS, et al. Oral and topical treatment of painful diabetic polyneuropathy: practice guideline update summary: report of the AAN Guideline Subcommittee. Neurology. 2022; 98:31–43.
Article83. Tesfaye S, Wilhelm S, Lledo A, Schacht A, Tolle T, Bouhassira D, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination?: the “COMBO-DN study”: a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013; 154:2616–25.84. Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009; 374:1252–61.
Article85. Holbech JV, Bach FW, Finnerup NB, Brosen K, Jensen TS, Sindrup SH. Imipramine and pregabalin combination for painful polyneuropathy: a randomized controlled trial. Pain. 2015; 156:958–66.86. Tesfaye S, Sloan G, Petrie J, White D, Bradburn M, Julious S, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022; 400:680–90.
Article87. Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain. 2003; 4:109–21.
Article88. Dubinsky RM, Miyasaki J. Assessment: efficacy of transcutaneous electric nerve stimulation in the treatment of pain in neurologic disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010; 74:173–6.
Article89. Jin DM, Xu Y, Geng DF, Yan TB. Effect of transcutaneous electrical nerve stimulation on symptomatic diabetic peripheral neuropathy: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2010; 89:10–5.
Article90. Gossrau G, Wahner M, Kuschke M, Konrad B, Reichmann H, Wiedemann B, et al. Microcurrent transcutaneous electric nerve stimulation in painful diabetic neuropathy: a randomized placebo-controlled study. Pain Med. 2011; 12:953–60.
Article91. Johnson MI, Bjordal JM. Transcutaneous electrical nerve stimulation for the management of painful conditions: focus on neuropathic pain. Expert Rev Neurother. 2011; 11:735–53.
Article92. Moran F, Leonard T, Hawthorne S, Hughes CM, McCrumGardner E, Johnson MI, et al. Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. J Pain. 2011; 12:929–35.
Article93. Walsh DM, Howe TE, Johnson MI, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database Syst Rev. 2009; 2:CD006142.
Article94. Forst T, Nguyen M, Forst S, Disselhoff B, Pohlmann T, Pfutzner A. Impact of low frequency transcutaneous electrical nerve stimulation on symptomatic diabetic neuropathy using the new Salutaris device. Diabetes Nutr Metab. 2004; 17:163–8.95. Upton GA, Tinley P, Al-Aubaidy H, Crawford R. The influence of transcutaneous electrical nerve stimulation parameters on the level of pain perceived by participants with painful diabetic neuropathy: a crossover study. Diabetes Metab Syndr. 2017; 11:113–8.
Article96. Katz N, Dworkin RH, North R, Thomson S, Eldabe S, Hayek SM, et al. Research design considerations for randomized controlled trials of spinal cord stimulation for pain: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials/Institute of Neuromodulation/International Neuromodulation Society recommendations. Pain. 2021; 162:1935–56.
Article97. Barchini J, Tchachaghian S, Shamaa F, Jabbur SJ, Meyerson BA, Song Z, et al. Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience. 2012; 215:196–208.
Article98. Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med. 2006; 7(Suppl 1):S14–26.
Article99. Stancak A, Kozak J, Vrba I, Tintera J, Vrana J, Polacek H, et al. Functional magnetic resonance imaging of cerebral activation during spinal cord stimulation in failed back surgery syndrome patients. Eur J Pain. 2008; 12:137–48.100. Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014; 17:515–50.
Article101. de Vos CC, Meier K, Zaalberg PB, Nijhuis HJ, Duyvendak W, Vesper J, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain. 2014; 155:2426–31.
Article102. Slangen R, Schaper NC, Faber CG, Joosten EA, Dirksen CD, van Dongen RT, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective twocenter randomized controlled trial. Diabetes Care. 2014; 37:3016–24.
Article103. Petersen EA, Stauss TG, Scowcroft JA, Brooks ES, White JL, Sills SM, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA Neurol. 2021; 78:687–98.104. Duarte RV, Nevitt S, Copley S, Maden M, de Vos CC, Taylor RS, et al. Systematic review and network meta-analysis of neurostimulation for painful diabetic neuropathy. Diabetes Care. 2022; 45:2466–75.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Intractable Painful Diabetic Peripheral Neuropathy: The Perspective of a Pain Specialist
- Diabetic Neuropathy
- Computerized Dynamic Posturography and Diabetic Peripheral Neuropathy
- Tarsal Tunnel Syndrome Combined with Diabetic Neuropathy
- Therapeutic alternatives in painful diabetic neuropathy: a meta-analysis of randomized controlled trials