Restor Dent Endod.
2021 May;46(2):e26. 10.5395/rde.2021.46.e26.
Cryopreservation of mesenchymal stem cells derived from dental pulp: a systematic review
- Affiliations
-
- 1Department of Restorative Dentistry, Universidade Federal do Paraná, Curitiba/PR, Brazil
- 2Department of Dentistry, Universidade Federal dos Vales do Jequitinhonha e Mucuri, Diamantina/MG, Brazil
- KMID: 2548070
- DOI: http://doi.org/10.5395/rde.2021.46.e26
Abstract
Objectives
The aim of the present systematic review was to investigate the cryopreservation process of dental pulp mesenchymal stromal cells and whether cryopreservation is effective in promoting cell viability and recovery.
Materials and Methods
This systematic review was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the research question was determined using the population, exposure, comparison, and outcomes strategy. Electronic searches were conducted in the PubMed, Cochrane Library, Science Direct, LILACS, and SciELO databases and in the gray literature (dissertations and thesis databases and Google Scholar) for relevant articles published up to March 2019. Clinical trial studies performed with dental pulp of human permanent or primary teeth, containing concrete information regarding the cryopreservation stages, and with cryopreservation performed for a period of at least 1 week were included in this study.
Results
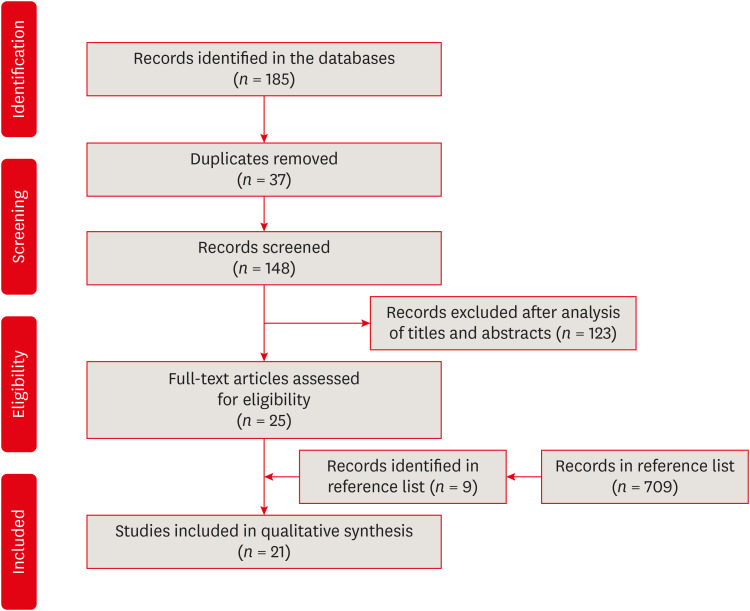
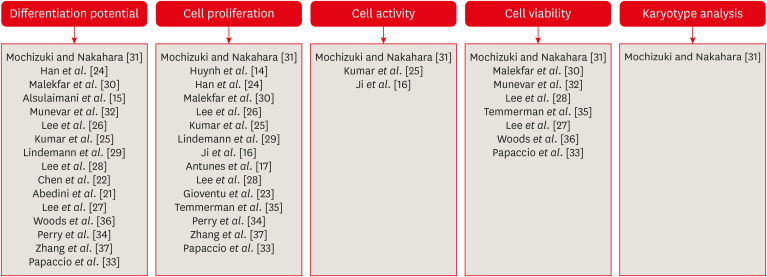
The search strategy resulted in the retrieval of 185 publications. After the application of the eligibility criteria, 21 articles were selected for a qualitative analysis.
Conclusions
The cryopreservation process must be carried out in 6 stages: tooth disinfection, pulp extraction, cell isolation, cell proliferation, cryopreservation, and thawing. In addition, it can be inferred that the use of dimethyl sulfoxide, programmable freezing, and storage in liquid nitrogen are associated with a high rate of cell viability after thawing and a high rate of cell proliferation in both primary and permanent teeth.
Figure
Reference
-
1. Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang J, Xu GT, Liang A, Liu S. Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells. 2015; 33:627–638. PMID: 25447379.
Article2. Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med. 2015; 9:1205–1216. PMID: 24850632.
Article3. Kawashima N, Noda S, Yamamoto M, Okiji T. Properties of dental pulp-derived mesenchymal stem cells and the effects of culture conditions. J Endod. 2017; 43:S31–S34. PMID: 28781092.
Article4. Mayo V, Sawatari Y, Huang CY, Garcia-Godoy F. Neural crest-derived dental stem cells--where we are and where we are going. J Dent. 2014; 42:1043–1051. PMID: 24769107.
Article5. Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res. 2014; 78:16–20. PMID: 24252618.
Article6. Wang J, Ma H, Jin X, Hu J, Liu X, Ni L, Ma PX. The effect of scaffold architecture on odontogenic differentiation of human dental pulp stem cells. Biomaterials. 2011; 32:7822–7830. PMID: 21663962.
Article7. Nakashima M, Iohara K. Mobilized dental pulp stem cells for pulp regeneration: initiation of clinical trial. J Endod. 2014; 40:S26–S32. PMID: 24698690.
Article8. Rosa V, Zhang Z, Grande RH, Nör JE. Dental pulp tissue engineering in full-length human root canals. J Dent Res. 2013; 92:970–975. PMID: 24056227.
Article9. Saghiri MA, Asatourian A, Sorenson CM, Sheibani N. Role of angiogenesis in endodontics: contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. J Endod. 2015; 41:797–803. PMID: 25649306.
Article10. Chamieh F, Collignon AM, Coyac BR, Lesieur J, Ribes S, Sadoine J, Llorens A, Nicoletti A, Letourneur D, Colombier ML, Nazhat SN, Bouchard P, Chaussain C, Rochefort GY. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci Rep. 2016; 6:38814. PMID: 27934940.
Article11. Estrela C, Alencar AH, Kitten GT, Vencio EF, Gava E. Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration. Braz Dent J. 2011; 22:91–98. PMID: 21537580.
Article12. Martin-Piedra MA, Garzon I, Oliveira AC, Alfonso-Rodriguez CA, Carriel V, Scionti G, Alaminos M. Cell viability and proliferation capability of long-term human dental pulp stem cell cultures. Cytotherapy. 2014; 16:266–277. PMID: 24438904.
Article13. Pegg DE. Principles of cryopreservation. Methods Mol Biol. 2007; 368:39–57. PMID: 18080461.
Article14. Huynh NC, Le SH, Doan VN, Ngo LT, Tran HL. Simplified conditions for storing and cryopreservation of dental pulp stem cells. Arch Oral Biol. 2017; 84:74–81. PMID: 28957734.
Article15. Alsulaimani RS, Ajlan SA, Aldahmash AM, Alnabaheen MS, Ashri NY. Isolation of dental pulp stem cells from a single donor and characterization of their ability to differentiate after 2 years of cryopreservation. Saudi Med J. 2016; 37:551–560. PMID: 27146619.
Article16. Ji EH, Song JS, Kim SO, Jeon M, Choi BJ, Lee JH. Viability of pulp stromal cells in cryopreserved deciduous teeth. Cell Tissue Bank. 2014; 15:67–74. PMID: 23670172.
Article17. Antunes FG. Atividade biológica de células-tronco da polpa de dentes decíduos humanos submetidas à criopreservação. Natal: Universidade Federal do Rio Grande do Norte;2013.18. Jesus AA, Soares MB, Soares AP, Nogueira RC, Guimarães ET, Araújo TM, Santos RR. Coleta e cultura de células-tronco obtidas da polpa de dentes decíduos: técnica e relato de caso clínico. Dental Press J Orthod. 2011; 16:111–118.
Article19. Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In : Aromataris E, Munn Z, editors. Joanna Briggs Institute reviewer's manual. Adelaide: JBI;2017.20. Lima IF, de Andrade Vieira W, de Macedo Bernardino Í, Costa PA, Lima AP, Pithon MM, Paranhos LR. Influence of reminder therapy for controlling bacterial plaque in patients undergoing orthodontic treatment: A systematic review and meta-analysis. Angle Orthod. 2018; 88:483–493. PMID: 29664334.
Article21. Abedini S, Kaku M, Kawata T, Koseki H, Kojima S, Sumi H, Motokawa M, Fujita T, Ohtani J, Ohwada N, Tanne K. Effects of cryopreservation with a newly-developed magnetic field programmed freezer on periodontal ligament cells and pulp tissues. Cryobiology. 2011; 62:181–187. PMID: 21397593.
Article22. Chen YK, Huang AH, Chan AW, Shieh TY, Lin LM. Human dental pulp stem cells derived from different cryopreservation methods of human dental pulp tissues of diseased teeth. J Oral Pathol Med. 2011; 40:793–800. PMID: 21518005.
Article23. Gioventù S, Andriolo G, Bonino F, Frasca S, Lazzari L, Montelatici E, Santoro F, Rebulla P. A novel method for banking dental pulp stem cells. Transfus Apheresis Sci. 2012; 47:199–206.
Article24. Han YJ, Kang YH, Shivakumar SB, Bharti D, Son YB, Choi YH, Park WU, Byun JH, Rho GJ, Park BW. Stem cells from cryopreserved human dental pulp tissues sequentially differentiate into definitive endoderm and hepatocyte-like cells in vitro . Int J Med Sci. 2017; 14:1418–1429. PMID: 29200956.
Article25. Kumar A, Bhattacharyya S, Rattan V. Effect of uncontrolled freezing on biological characteristics of human dental pulp stem cells. Cell Tissue Bank. 2015; 16:513–522. PMID: 25663639.
Article26. Lee HS, Jeon M, Kim SO, Kim SH, Lee JH, Ahn SJ, Shin Y, Song JS. Characteristics of stem cells from human exfoliated deciduous teeth (SHED) from intact cryopreserved deciduous teeth. Cryobiology. 2015; 71:374–383. PMID: 26506257.
Article27. Lee SY, Chiang PC, Tsai YH, Tsai SY, Jeng JH, Kawata T, Huang HM. Effects of cryopreservation of intact teeth on the isolated dental pulp stem cells. J Endod. 2010; 36:1336–1340. PMID: 20647092.
Article28. Lee SY, Huang GW, Shiung JN, Huang YH, Jeng JH, Kuo TF, Yang JC, Yang WC. Magnetic cryopreservation for dental pulp stem cells. Cells Tissues Organs. 2012; 196:23–33. PMID: 22285908.
Article29. Lindemann D, Werle SB, Steffens D, Garcia-Godoy F, Pranke P, Casagrande L. Effects of cryopreservation on the characteristics of dental pulp stem cells of intact deciduous teeth. Arch Oral Biol. 2014; 59:970–976. PMID: 24949827.
Article30. Malekfar A, Valli KS, Kanafi MM, Bhonde RR. Isolation and characterization of human dental pulp stem cells from cryopreserved pulp tissues obtained from teeth with irreversible pulpitis. J Endod. 2016; 42:76–81. PMID: 26577871.
Article31. Mochizuki M, Nakahara T. Establishment of xenogeneic serum-free culture methods for handling human dental pulp stem cells using clinically oriented in-vitro and in-vivo conditions. Stem Cell Res Ther. 2018; 9:25. PMID: 29394956.
Article32. Munévar JC, Gutiérrez N, Jiménez NT, Lafaurie GI. Evaluation of two human dental pulp stem cell cryopreservation methods. Acta Odontol Latinoam. 2015; 28:114–121. PMID: 26355880.33. Papaccio G, Graziano A, d'Aquino R, Graziano MF, Pirozzi G, Menditti D, De Rosa A, Carinci F, Laino G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006; 208:319–325. PMID: 16622855.
Article34. Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008; 14:149–156. PMID: 18489245.
Article35. Temmerman L, Beele H, Dermaut LR, Van Maele G, De Pauw GA. Influence of cryopreservation on the pulpal tissue of immature third molars in vitro. Cell Tissue Bank. 2010; 11:281–289. PMID: 19685168.
Article36. Woods EJ, Perry BC, Hockema JJ, Larson L, Zhou D, Goebel WS. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology. 2009; 59:150–157. PMID: 19538953.
Article37. Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006; 12:2813–2823. PMID: 17518650.
Article38. Hilkens P, Driesen RB, Wolfs E, Gervois P, Vangansewinkel T, Ratajczak J, Dillen Y, Bronckaers A, Lambrichts I. Cryopreservation and Banking of Dental Stem Cells. Adv Exp Med Biol. 2016; 951:199–235. PMID: 27837566.
Article39. Oh YH, Che ZM, Hong JC, Lee EJ, Lee SJ, Kim J. Cryopreservation of human teeth for future organization of a tooth bank--a preliminary study. Cryobiology. 2005; 51:322–329. PMID: 16297377.
Article40. Khademi AA, Saei S, Mohajeri MR, Mirkheshti N, Ghassami F, Torabi nia N, Alavi SA. A new storage medium for an avulsed tooth. J Contemp Dent Pract. 2008; 9:25–32.
Article41. Eubanks EJ, Tarle SA, Kaigler D. Tooth storage, dental pulp stem cell isolation, and clinical scale expansion without animal serum. J Endod. 2014; 40:652–657. PMID: 24767559.
Article42. Salehinejad P, Alitheen NB, Ali AM, Omar AR, Mohit M, Janzamin E, Samani FS, Torshizi Z, Nematollahi-Mahani SN. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton's jelly. In Vitro Cell Dev Biol Anim. 2012; 48:75–83. PMID: 22274909.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of Human Dental Pulp Cells from Supernumerary Teeth by Using Flow Cytometry Analysis
- Cryopreservation of dental tissue and subsequent isolation of mesenchymal stem cells
- Dental Pulp Stem Cells and Current in vivo Approaches to Study Dental Pulp Stem Cells in Pulp Injury and Regeneration
- Modulation of osteoblastic/odontoblastic differentiation of adult mesenchymal stem cells through gene introduction: a brief review
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells