Clin Endosc.
2023 Nov;56(6):795-801. 10.5946/ce.2022.289.
The efficacy of a novel integrated outside biliary stent and nasobiliary drainage catheter system for acute cholangitis: a single center pilot study
- Affiliations
-
- 1Department of Gastroenterology, Saiseikai Kawaguchi General Hospital, Kawaguchi, Japan
- 2Department of Gastroenterology, Dokkyo Medical University Saitama Medical Center, Koshigaya, Japan
- KMID: 2547902
- DOI: http://doi.org/10.5946/ce.2022.289
Abstract
- Background/Aims
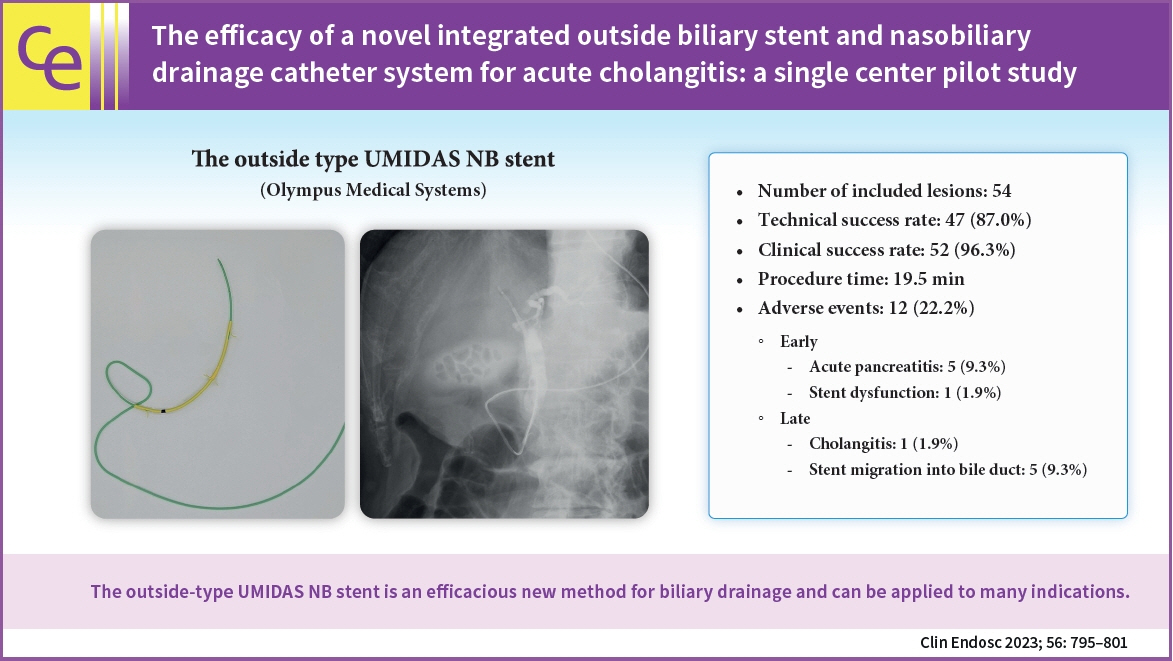
Endoscopic biliary drainage is the gold standard treatment for cholangitis. The two methods of biliary drainage are endoscopic biliary stenting and nasobiliary drainage. A novel integrated outside biliary stent and nasobiliary drainage catheter system (UMIDAS NB stent; Olympus Medical Systems) was recently developed. In this study, we evaluated the efficacy of this stent in the treatment of cholangitis caused by common bile duct stones or distal bile duct strictures.
Methods
We conducted a retrospective pilot study by examining the medical records of patients who required endoscopic biliary drainage for cholangitis due to common bile duct stones or distal bile duct strictures, and who were treated with a UMIDAS NB stent, between December 2021 and July 2022.
Results
Records of 54 consecutive patients were reviewed. Technical and clinical success rates were 47/54 (87.0%) and 52/54 (96.3%), respectively. Adverse events were observed in 12 patients, with six patients experiencing pancreatitis as an adverse event, following endoscopic retrograde cholangiopancreatography (ERCP). Regarding late adverse events, five cases of biliary stent migration into the bile duct were observed. Disease-related death occurred in one patient.
Conclusions
The outside-type UMIDAS NB stent is an efficacious new method for biliary drainage and can be applied to many indications.
Figure
Reference
-
1. Lai EC, Mok FP, Tan ES, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992; 326:1582–1586.
Article2. Leung JW, Chung SC, Sung JJ, et al. Urgent endoscopic drainage for acute suppurative cholangitis. Lancet. 1989; 1:1307–1309.
Article3. Mukai S, Itoi T, Baron TH, et al. Indications and techniques of biliary drainage for acute cholangitis in updated Tokyo Guidelines 2018. J Hepatobiliary Pancreat Sci. 2017; 24:537–549.
Article4. Ramchandani M, Pal P, Reddy DN. Endoscopic management of acute cholangitis as a result of common bile duct stones. Dig Endosc. 2017; 29 Suppl 2:78–87.
Article5. Sharma BC, Kumar R, Agarwal N, et al. Endoscopic biliary drainage by nasobiliary drain or by stent placement in patients with acute cholangitis. Endoscopy. 2005; 37:439–443.
Article6. Park SY, Park CH, Cho SB, et al. The safety and effectiveness of endoscopic biliary decompression by plastic stent placement in acute suppurative cholangitis compared with nasobiliary drainage. Gastrointest Endosc. 2008; 68:1076–1080.
Article7. Kawashima H, Itoh A, Ohno E, et al. Is nasobiliary drainage unnecessary for drainage of acute suppurative cholangitis?: our experience. Dig Endosc. 2010; 22 Suppl 1:S118–S122.
Article8. Lee DW, Chan AC, Lam YH, et al. Biliary decompression by nasobiliary catheter or biliary stent in acute suppurative cholangitis: a prospective randomized trial. Gastrointest Endosc. 2002; 56:361–365.
Article9. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.
Article10. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655.
Article11. Masci E, Toti G, Mariani A, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001; 96:417–423.
Article12. Vandervoort J, Soetikno RM, Tham TC, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002; 56:652–656.
Article13. Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001; 54:425–434.
Article14. Kawakami H, Itoi T. A novel integrated inside biliary stent and nasobiliary drainage catheter system for biliary drainage (with video). J Hepatobiliary Pancreat Sci. 2020; 27:149–150.
Article15. Iwano K, Kuriyama K, Yazumi S. Multi-stent technique using a novel integrated inside biliary stent and nasobiliary drainage catheter system for malignant perihilar biliary obstruction. Dig Endosc. 2022; 34:e46–e47.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Emergency Nasobiliary Drainage in Acute Suppurative Cholangitis
- The Clinical Usefulness of Simultaneous Placement of Double Endoscopic Nasobiliary Biliary Drainage
- Percutaneous Transhepatic Biliary Drainage Using a Ligated Catheter for Recurrent Catheter Obstruction: Antireflux Technique
- A Case of Sigmoid Colonic Perforation due to Migration of a Plastic Stent for Endoscopic Retrograde Biliary Drainage
- Diagnosis and Treatment of Acute Cholangitis