Cancer Res Treat.
2023 Oct;55(4):1346-1354. 10.4143/crt.2022.1436.
Safety and Tolerability of Weekly Genexol-PM, a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel, with Carboplatin in Gynecologic Cancer: A Phase I Study
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Obstetrics and Gynecology, GangNeung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea
- KMID: 2547808
- DOI: http://doi.org/10.4143/crt.2022.1436
Abstract
- Purpose
This phase I study was conducted to determine the maximum tolerated dose and the recommended phase II dose of weekly administered Genexol-PM combined with carboplatin in patients with gynecologic cancer.
Materials and Methods
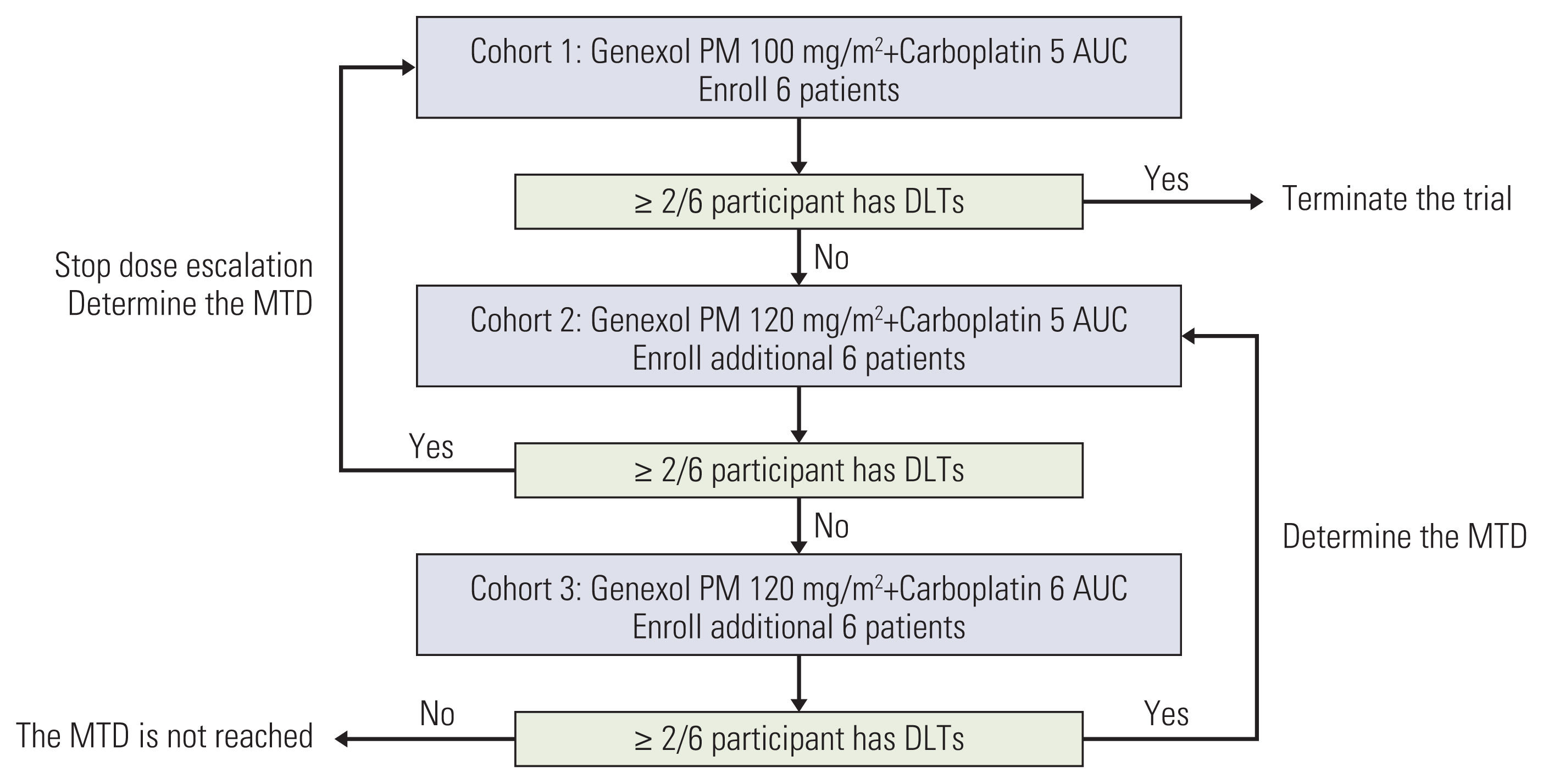
This open-label, phase I, dose-escalation study of weekly Genexol-PM included 18 patients with gynecologic cancer, who were equally divided into three cohorts of dose levels. Cohort 1 received 100 mg/m2 Genexol-PM and 5 area under the curve (AUC) carboplatin, cohort 2 received 120 mg/m2 Genexol-PM and 5 AUC carboplatin, and cohort 3 received 120 mg/m2 Genexol-PM and 6 AUC carboplatin. The safety and efficacy of each dose were analyzed for each cohort.
Results
Of the 18 patients, 11 patients were newly diagnosed and seven patients were recurrent cases. No dose-limiting toxicity was observed. The maximum tolerated dose was not defined, but a dose up to 120 mg/m2 of Genexol-PM in combination with AUC 5-6 of carboplatin could be recommended for a phase II study. In this intention-to-treat population, five patients dropped out of the study (carboplatin-related hypersensitivity, n=1; refusal of consent, n=4). Most patients (88.9%) with adverse events recovered without sequelae, and no treatment-related death occurred. The overall response rate of weekly Genexol-PM in combination with carboplatin was 72.2%.
Conclusion
Weekly administered Genexol-PM with carboplatin demonstrated an acceptable safety profile in gynecologic cancer pati-ents. The recommended phase II dose of weekly Genexol-PM is up to 120 mg/m2 when combined with carboplatin.
Figure
Reference
-
References
1. Ovarian cancer (version 1.2022) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2022 [cited 2022 Jan 18]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf .2. Uterine neoplasms (version 1.2022) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2022 [cited 2021 Nov 4]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf .3. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003; 21:3194–200.
Article4. du Bois A, Neijt JP, Thigpen JT. First line chemotherapy with carboplatin plus paclitaxel in advanced ovarian cancer: a new standard of care? Ann Oncol. 1999; 10(Suppl 1):35–41.5. Miller DS, Filiaci VL, Mannel RS, Cohn DE, Matsumoto T, Tewari KS, et al. Carboplatin and paclitaxel for advanced endometrial cancer: final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG0209). J Clin Oncol. 2020; 38:3841–50.
Article6. Banerjee S, Moore KN, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021; 22:1721–31.
Article7. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020; 38:1–10.
Article8. Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018; 81:17–38.
Article9. Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009; 374:1331–8.
Article10. Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013; 14:1020–6.
Article11. Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S, et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014; 15:396–405.
Article12. Mathew AE, Mejillano MR, Nath JP, Himes RH, Stella VJ. Synthesis and evaluation of some water-soluble prodrugs and derivatives of taxol with antitumor activity. J Med Chem. 1992; 35:145–51.
Article13. Lee SW, Kim YM, Kim YT, Kang SB. An open-label, multicenter, phase I trial of a cremophor-free, polymeric micelle formulation of paclitaxel combined with carboplatin as a first-line treatment for advanced ovarian cancer: a Korean Gynecologic Oncology Group study (KGOG-3016). J Gynecol Oncol. 2017; 28:e26.
Article14. Kloover JS, den Bakker MA, Gelderblom H, van Meerbeeck JP. Fatal outcome of a hypersensitivity reaction to paclitaxel: a critical review of premedication regimens. Br J Cancer. 2004; 90:304–5.
Article15. Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Wan Kim S, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001; 72:191–202.
Article16. Lee SW, Kim YM, Cho CH, Kim YT, Kim SM, Hur SY, et al. An open-label, randomized, parallel, phase II trial to evaluate the efficacy and safety of a cremophor-free polymeric micelle formulation of paclitaxel as first-line treatment for ovarian cancer: a Korean Gynecologic Oncology Group Study (KGOG-3021). Cancer Res Treat. 2018; 50:195–203.
Article17. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.18. Rustin GJ. Use of CA-125 to assess response to new agents in ovarian cancer trials. J Clin Oncol. 2003; 21:187s–93s.
Article19. Clamp AR, James EC, McNeish IA, Dean A, Kim JW, O’Donnell DM, et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet. 2019; 394:2084–95.
Article20. Chan JK, Brady MF, Penson RT, Huang H, Birrer MJ, Walker JL, et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med. 2016; 374:738–48.
Article21. Ba Y, Shi Y, Jiang W, Feng J, Cheng Y, Xiao L, et al. Current management of chemotherapy-induced neutropenia in adults: key points and new challenges: Committee of Neoplastic Supportive-Care (CONS), China Anti-Cancer Association Committee of Clinical Chemotherapy, China Anti-Cancer Association. Cancer Biol Med. 2020; 17:896–909.
Article22. Scripture CD, Figg WD, Sparreboom A. Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol. 2006; 4:165–72.
Article23. Boulanger J, Boursiquot JN, Cournoyer G, Lemieux J, Masse MS, Almanric K, et al. Management of hypersensitivity to platinum- and taxane-based chemotherapy: cepo review and clinical recommendations. Curr Oncol. 2014; 21:e630–41.
Article24. Shawky H, Tawfik H, Hewidy M. Weekly dose-dense paclitaxel and carboplatin in recurrent ovarian carcinoma: a phase II trial. J Egypt Natl Canc Inst. 2014; 26:139–45.
Article25. Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Des Devel Ther. 2015; 9:3767–77.
Article26. Lim WT, Tan EH, Toh CK, Hee SW, Leong SS, Ang PC, et al. Phase I pharmacokinetic study of a weekly liposomal paclitaxel formulation (Genexol-PM) in patients with solid tumors. Ann Oncol. 2010; 21:382–8.
Article27. Kim JY, Do YR, Song HS, Cho YY, Ryoo HM, Bae SH, et al. Multicenter phase II clinical trial of Genexol-PM(R) with gemcitabine in advanced biliary tract cancer. Anticancer Res. 2017; 37:1467–73.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Open-Label, Randomized, Parallel, Phase II Trial to Evaluate the Efficacy and Safety of a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel as First-Line Treatment for Ovarian Cancer: A Korean Gynecologic Oncology Group Study (KGOG-3021)
- An open-label, multicenter, phase I trial of a cremophor-free, polymeric micelle formulation of paclitaxel combined with carboplatin as a first-line treatment for advanced ovarian cancer: a Korean Gynecologic Oncology Group study (KGOG-3016)
- Non-convulsive seizure related to Cremophor ELâ„¢-free, polymeric micelle formulation of paclitaxel: a case report
- An Open-Label, Randomized, Parallel, Phase III Trial Evaluating the Efficacy and Safety of Polymeric Micelle-Formulated Paclitaxel Compared to Conventional Cremophor EL-Based Paclitaxel for Recurrent or Metastatic HER2-Negative Breast Cancer
- A Case of Ischemic Colitis Associated with Paclitaxel Loaded Polymeric Micelle (Genexol-PM(R)) Chemotherapy