Cancer Res Treat.
2023 Oct;55(4):1313-1320. 10.4143/crt.2023.452.
Analysis of Plasma Circulating Tumor DNA in Borderline Resectable Pancreatic Cancer Treated with Neoadjuvant Modified FOLFIRINOX: Clinical Relevance of DNA Damage Repair Gene Alteration Detection
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2547805
- DOI: http://doi.org/10.4143/crt.2023.452
Abstract
- Purpose
There are no reliable biomarkers to guide treatment for patients with borderline resectable pancreatic cancer (BRPC) in the neoadjuvant setting. We used plasma circulating tumor DNA (ctDNA) sequencing to search biomarkers for patients with BRPC receiving neoadjuvant mFOLFIRINOX in our phase 2 clinical trial (NCT02749136).
Materials and Methods
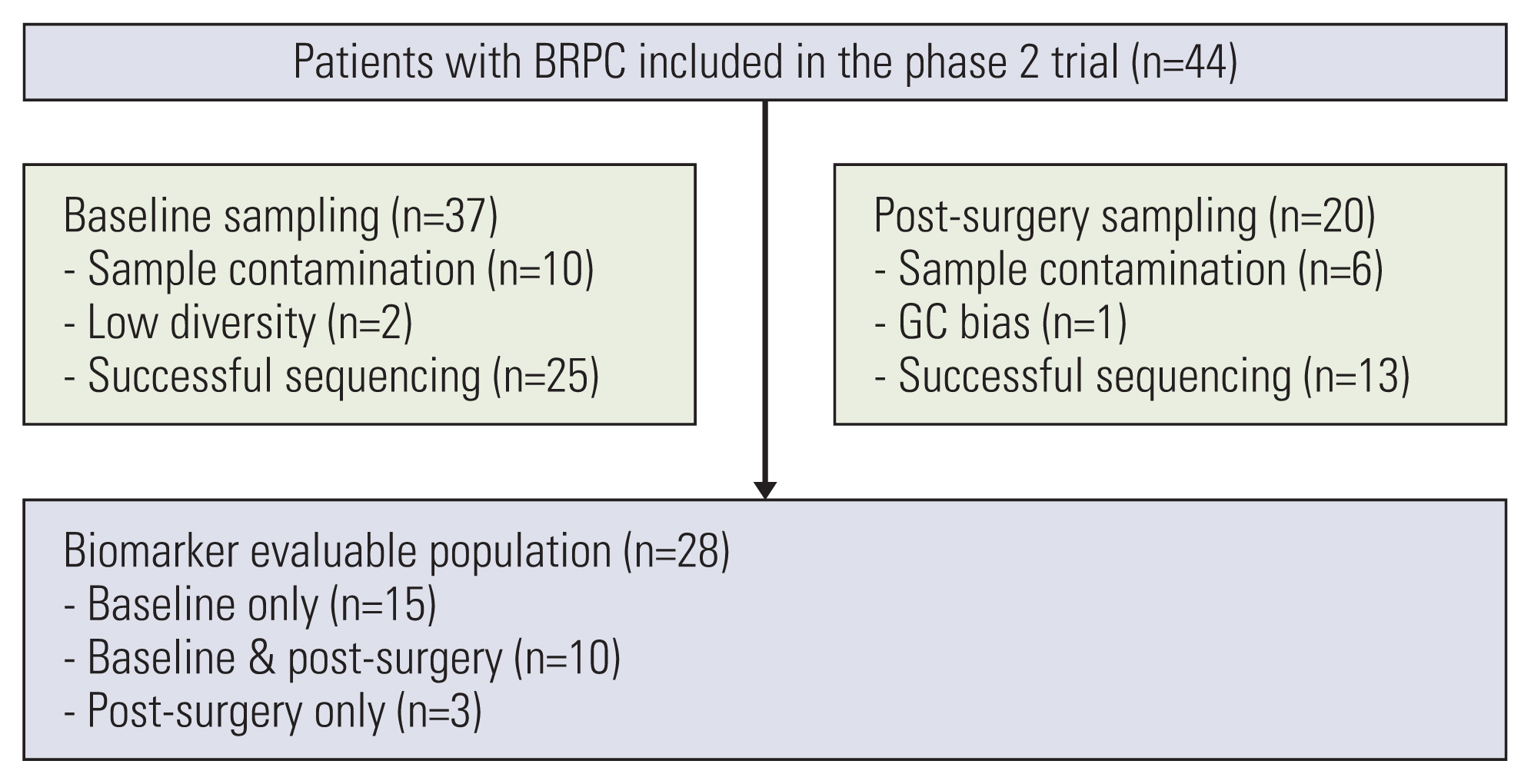
Among the 44 patients enrolled in the trial, patients with plasma ctDNA sequencing at baseline or post-operation were included in this analysis. Plasma cell-free DNA isolation and sequencing were performed using the Guardant 360 assay. Detection of genomic alterations, including DNA damage repair (DDR) genes, were examined for correlations with survival.
Results
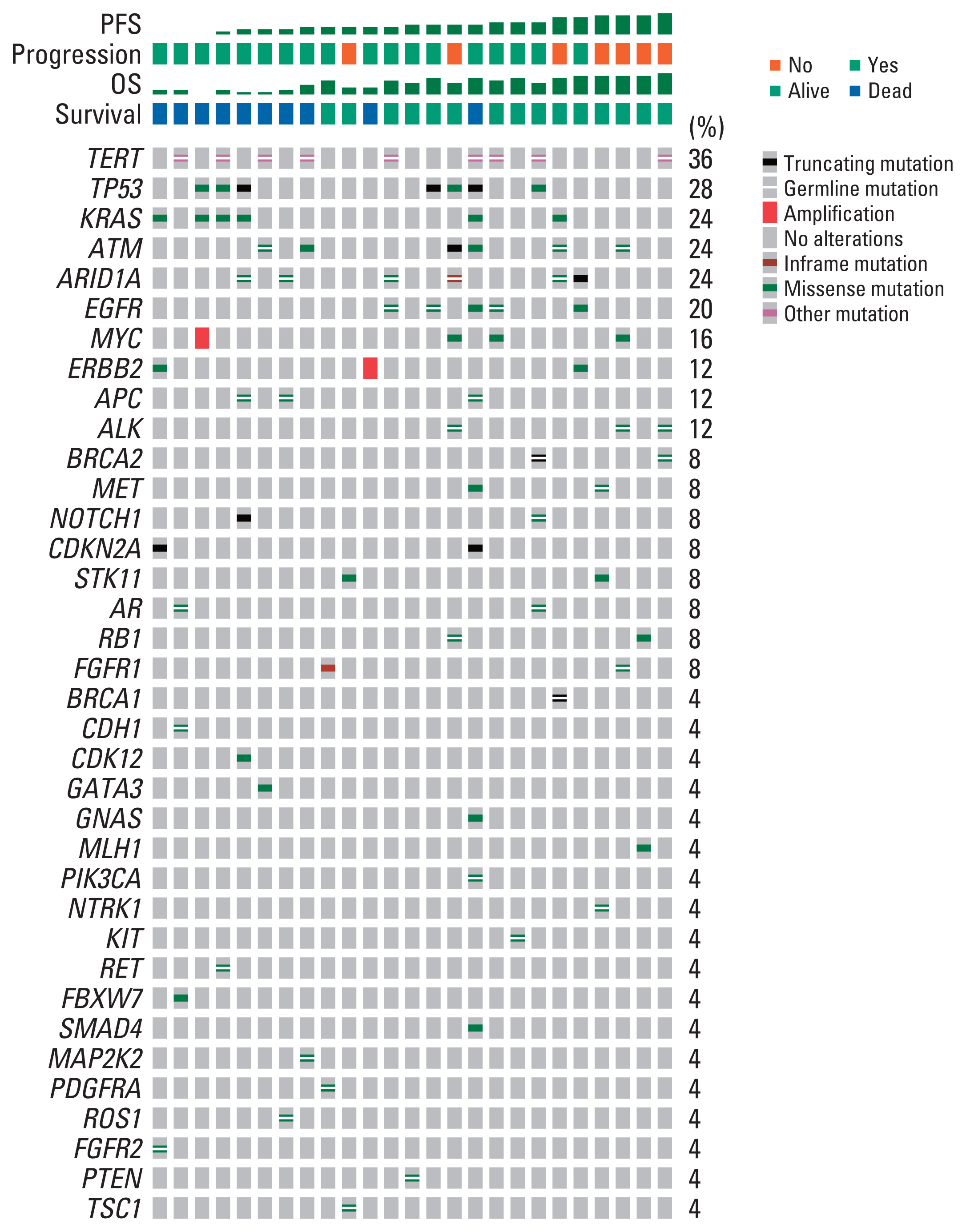
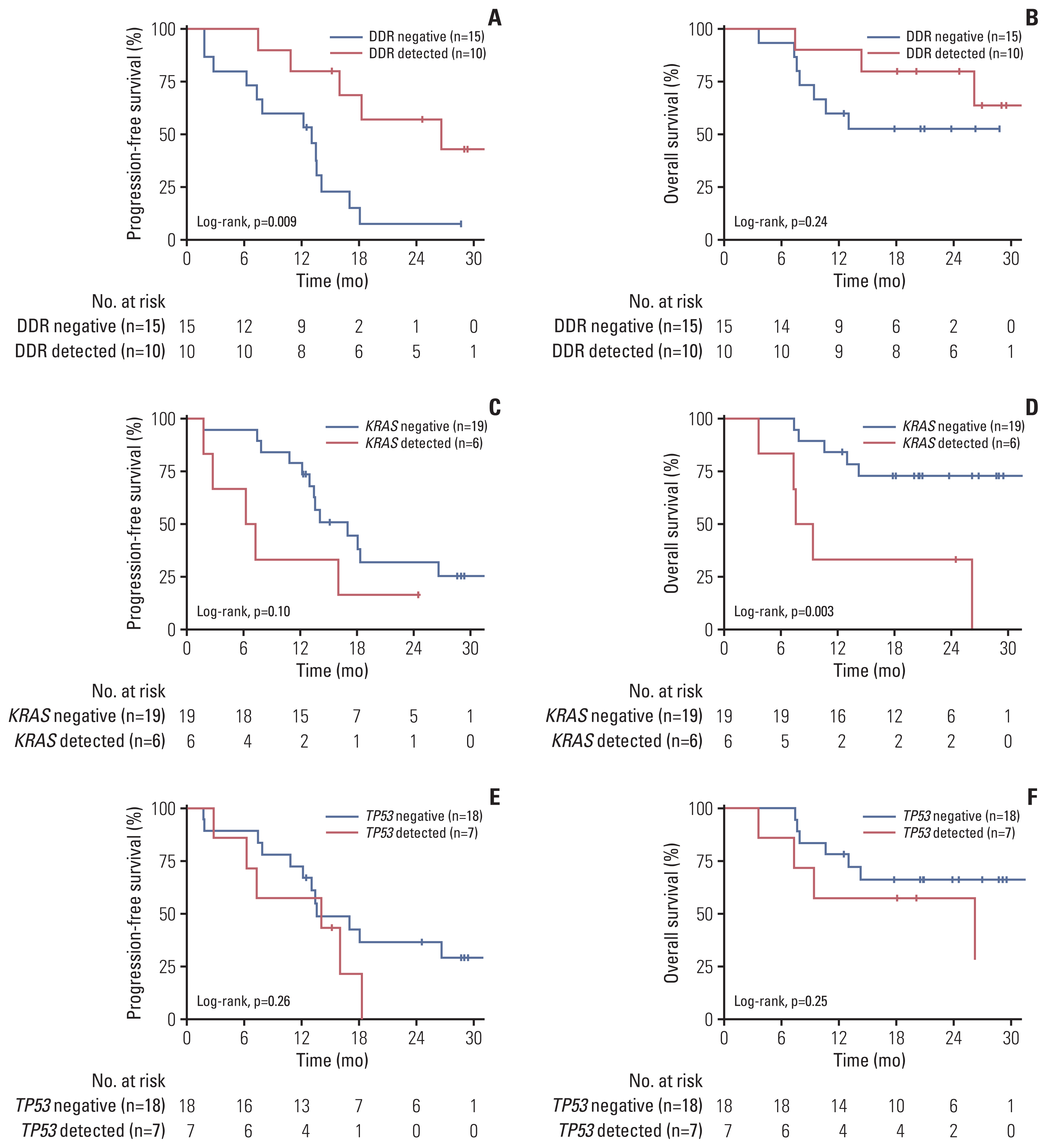
Among the 44 patients, 28 patients had ctDNA sequencing data qualified for the analysis and were included in this study. Among the 25 patients with baseline plasma ctDNA data, 10 patients (40%) had alterations of DDR genes detected at baseline, inclu-ding ATM, BRCA1, BRCA2 and MLH1, and showed significantly better progression-free survival than those without such DDR gene alterations detected (median, 26.6 vs. 13.5 months; log-rank p=0.004). Patients with somatic KRAS mutations detected at baseline (n=6) had significantly worse overall survival (median, 8.5 months vs. not applicable; log-rank p=0.003) than those without. Among 13 patients with post-operative plasma ctDNA data, eight patients (61.5%) had detectable somatic alterations.
Conclusion
Detection of DDR gene mutations from plasma ctDNA at baseline was associated with better survival outcomes of pati-ents with borderline resectable pancreatic ductal adenocarcinoma treated with neoadjuvant mFOLFIRINOX and may be a prognostic biomarker.
Keyword
Figure
Reference
-
References
1. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020; 395:2008–20.2. Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology. 2014; 146:291–304e1.3. Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010; 7:e1000267.4. National Comprehensive Cancer Network. Pancreatic adenocarcinoma (version 1.2021) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;c2021 [cited 2021 Mar 13]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf .5. Yoo C, Lee SS, Song KB, Jeong JH, Hyung J, Park DH, et al. Neoadjuvant modified FOLFIRINOX followed by postoperative gemcitabine in borderline resectable pancreatic adenocarcinoma: a Phase 2 study for clinical and biomarker analysis. Br J Cancer. 2020; 123:362–8.6. Hyung J, Lee SS, Hwang DW, Park JH, Kim KP, Yoo C. Current status and future perspective of neoadjuvant therapy in locally advanced and borderline resectable pancreatic adenocarcinoma: a narrative review. Chin Clin Oncol. 2022; 11:20.7. Wan JC, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, et al. Liquid biopsies come of age: towards imp-lementation of circulating tumour DNA. Nat Rev Cancer. 2017; 17:223–38.8. Russano M, Napolitano A, Ribelli G, Iuliani M, Simonetti S, Citarella F, et al. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: the potentiality of blood samples. J Exp Clin Cancer Res. 2020; 39:95.9. Faulkner LG, Howells LM, Pepper C, Shaw JA, Thomas AL. The utility of ctDNA in detecting minimal residual disease following curative surgery in colorectal cancer: a systematic review and meta-analysis. Br J Cancer. 2023; 128:297–309.10. Magbanua MJ, Swigart LB, Wu HT, Hirst GL, Yau C, Wolf DM, et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol. 2021; 32:229–39.11. Bernard V, Kim DU, San Lucas FA, Castillo J, Allenson K, Mulu FC, et al. Circulating nucleic acids are associated with outcomes of patients with pancreatic cancer. Gastroenterology. 2019; 156:108–18.12. Bhangu JS, Beer A, Mittlbock M, Tamandl D, Pulverer W, Schonthaler S, et al. Circulating free methylated tumor DNA markers for sensitive assessment of tumor burden and early response monitoring in patients receiving systemic chemotherapy for colorectal cancer liver metastasis. Ann Surg. 2018; 268:894–902.13. Wattenberg MM, Asch D, Yu S, O’Dwyer PJ, Domchek SM, Nathanson KL, et al. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer. 2020; 122:333–9.14. Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014; 111:1132–8.15. Huang R, Zhou PK. DNA damage repair: historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct Target Ther. 2021; 6:254.16. Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017; 32:185–203.17. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012; 2:401–4.18. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013; 6:pl1.19. Nakano Y, Kitago M, Matsuda S, Nakamura Y, Fujita Y, Imai S, et al. KRAS mutations in cell-free DNA from preoperative and postoperative sera as a pancreatic cancer marker: a retrospective study. Br J Cancer. 2018; 118:662–9.20. Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012; 481:287–94.21. Dilruba S, Kalayda GV. Platinum-based drugs: past, present and future. Cancer Chemother Pharmacol. 2016; 77:1103–24.22. Plimack ER, Dunbrack RL, Brennan TA, Andrake MD, Zhou Y, Serebriiskii IG, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder Cancer. Eur Urol. 2015; 68:959–67.23. Mota JM, Barnett E, Nauseef JT, Nguyen B, Stopsack KH, Wibmer A, et al. Platinum-based chemotherapy in metastatic prostate cancer with DNA repair gene alterations. JCO Precis Oncol. 2020; 4:355–66.24. Sehdev A, Gbolahan O, Hancock BA, Stanley M, Shahda S, Wan J, et al. Germline and somatic DNA damage repair gene mutations and overall survival in metastatic pancreatic adenocarcinoma patients treated with FOLFIRINOX. Clin Cancer Res. 2018; 24:6204–11.25. Pishvaian MJ, Blais EM, Brody JR, Rahib L, Lyons E, De Arbeloa P, et al. Outcomes in patients with pancreatic adenocarcinoma with genetic mutations in DNA damage response pathways: results from the know your tumor program. JCO Precis Oncol. 2019; 3:1–10.26. Luo J. KRAS mutation in pancreatic cancer. Semin Oncol. 2021; 48:10–8.27. Guo S, Shi X, Shen J, Gao S, Wang H, Shen S, et al. Preoperative detection of KRAS G12D mutation in ctDNA is a powerful predictor for early recurrence of resectable PDAC patients. Br J Cancer. 2020; 122:857–67.28. Earl J, Garcia-Nieto S, Martinez-Avila JC, Montans J, Sanjuanbenito A, Rodriguez-Garrote M, et al. Circulating tumor cells (Ctc) and kras mutant circulating free Dna (cfdna) detection in peripheral blood as biomarkers in patients diagnosed with exocrine pancreatic cancer. BMC Cancer. 2015; 15:797.29. Guven DC, Sahin TK, Yildirim HC, Aktepe OH, Dizdar O, Yalcin S. A systematic review and meta-analysis of the association between circulating tumor DNA (ctDNA) and prognosis in pancreatic cancer. Crit Rev Oncol Hematol. 2021; 168:103528.30. Chen L, Zhang Y, Cheng Y, Zhang D, Zhu S, Ma X. Prognostic value of circulating cell-free DNA in patients with pancreatic cancer: a systemic review and meta-analysis. Gene. 2018; 679:328–34.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant Therapy for Resectable or Borderline Resectable Pancreatic Cancer
- Neoadjuvant and Adjuvant Treatments for Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma: The Current Status of Pancreatic Ductal Adenocarcinoma Treatment in Japan
- Clinical Application of Circulating Tumor DNA Analysis
- Updates of Chemotherapy and Radiotherapy for Pancreatic Cancer
- Comparison of Clinical Outcomes of Borderline Resectable Pancreatic Cancer According to the Neoadjuvant Chemo-Regimens: Gemcitabine versus FOLFIRINOX