Cancer Res Treat.
2023 Oct;55(4):1113-1122. 10.4143/crt.2022.1651.
Individualized Concurrent Chemotherapy for Patients with Stage III-IVa Nasopharyngeal Carcinoma Receiving Neoadjuvant Chemotherapy Combined with Definitive Intensity-Modulated Radiotherapy
- Affiliations
-
- 1Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital, Fujian, China
- 2Department of Otolaryngology, Fujian Medical University Union Hospital, Fujian, China
- 3Key Laboratory of Ministry of Education for Gastrointestinal Cancer, Fujian Medical University, Fujian, China
- 4Department of Preventive Medicine, School of Public Health, Fujian Medical University, Fujian, China
- 5Fuzhou Center for Disease Control and Prevention, Fuzhou, Fujian, China
- 6Fujian Key Laboratory of Translational Cancer Medicine, Fujian, China
- KMID: 2547786
- DOI: http://doi.org/10.4143/crt.2022.1651
Abstract
- Purpose
This retrospective study aimed to re-evaluate the effect of concurrent chemotherapy in patients with locally advanced nasopharyngeal carcinoma (NPC) in the era of intensity-modulated radiotherapy (IMRT).
Materials and Methods
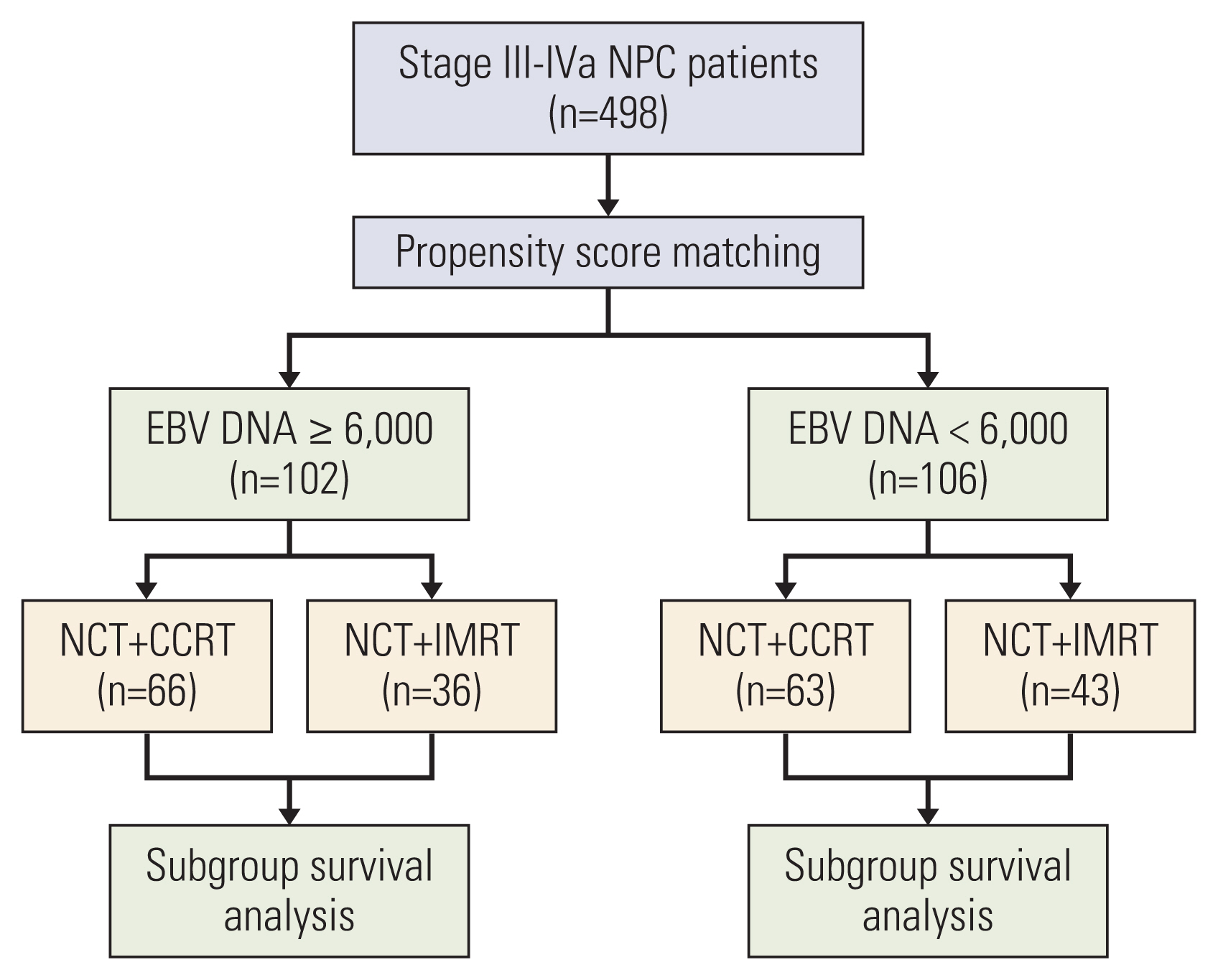
A total of 498 patients who received neoadjuvant chemotherapy (NCT) combined with concurrent chemoradiotherapy (CCRT) or IMRT were retrospectively reviewed. The distribution of baseline characteristics was balanced using propensity score matching. Additionally, the results of NCT+IMRT and NCT+CCRT were compared using Kaplan-Meier survival analysis, and differences in survival rates were analyzed using the log rank test.
Results
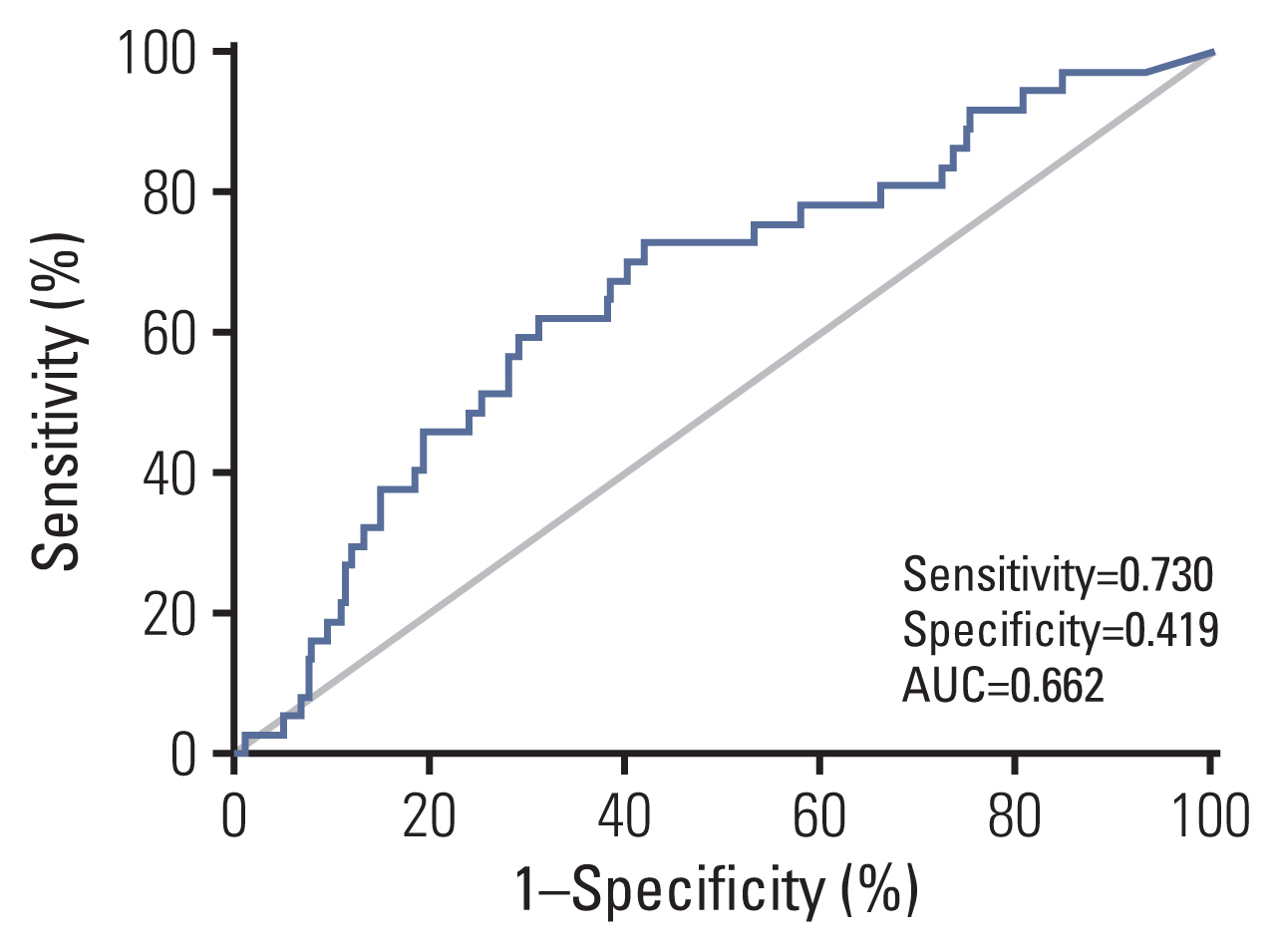
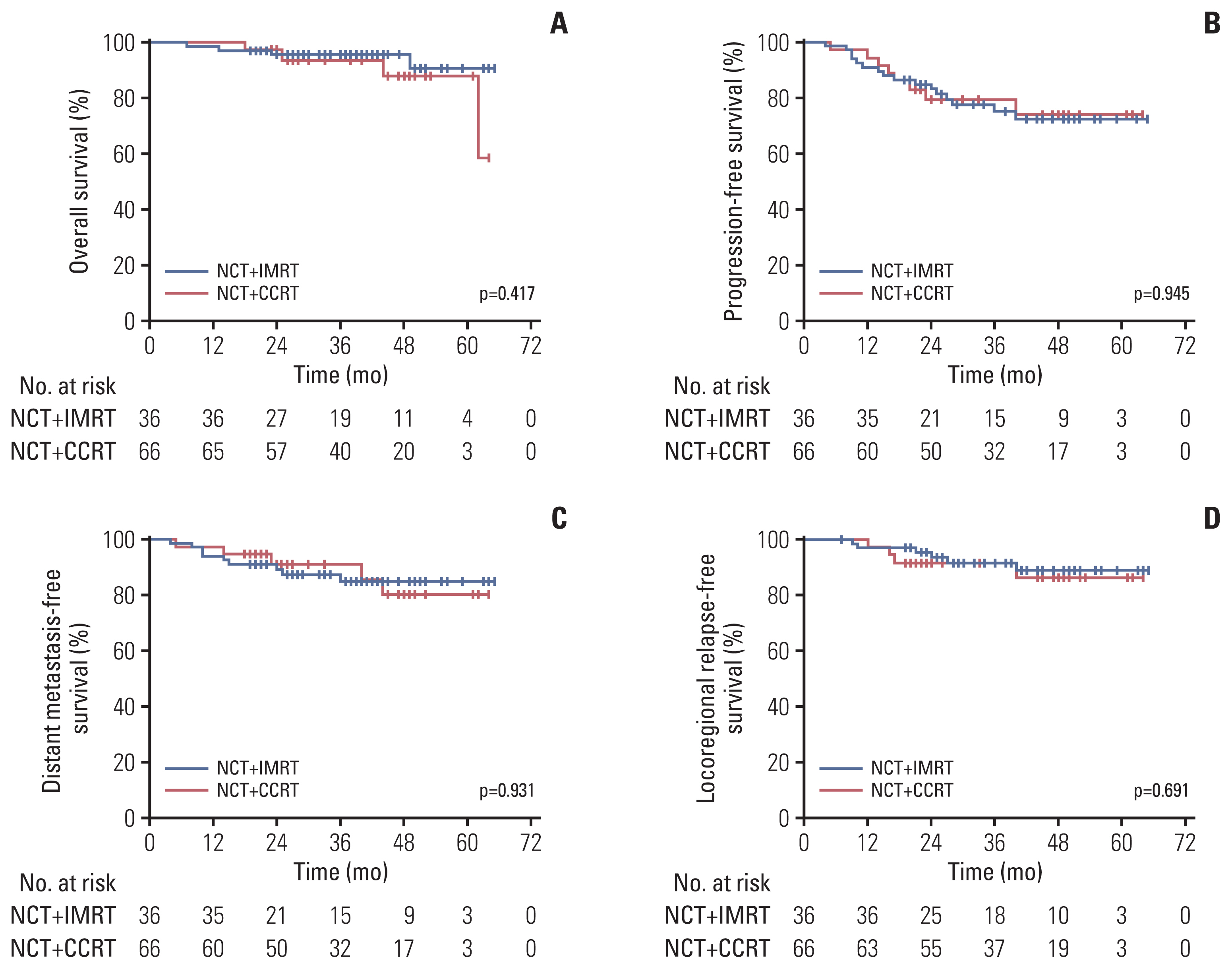
There were no significant differences in overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), and local progression-free survival (LRFS) between the two groups. Patients were further categorized into risk subgroups based on pretreatment Epstein-Barr virus (EBV) DNA cutoff values using receiver operating characteristic curve analysis. There were no statistically significant differences in OS, PFS, DMFS, and LRFS between patients who received NCT+CCRT and NCT+IMRT in the high-risk group. In the low-risk group, although there were no differences between NCT+CCRT and NCT+IMRT in OS, PFS, and LRFS, patients who received NCT+CCRT had better DMFS than those who received NCT+IMRT.
Conclusion
Pretreatment EBV DNA level can be used to individualize concurrent chemotherapy for patients with locally advanced NPC. Patients with low pretreatment EBV DNA levels may benefit from concurrent chemotherapy, whereas those with high levels may not. Other treatment modalities need to be explored for high-risk patients to improve their prognosis.
Keyword
Figure
Reference
-
References
1. Chen YP, Chan AT, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019; 394:64–80.2. Chua ML, Wee JTS, Hui EP, Chan AT. Nasopharyngeal carcinoma. Lancet. 2016; 387:1012–24.3. Ribassin-Majed L, Marguet S, Lee AW, Ng WT, Ma J, Chan AT, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol. 2017; 35:498–505.4. Chen YP, Ismaila N, Chua ML, Colevas AD, Haddad R, Huang SH, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II–IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021; 39:840–59.5. Sun XS, Liu SL, Luo MJ, Li XY, Chen QY, Guo SS, et al. The association between the development of radiation therapy, image technology, and chemotherapy, and the survival of patients with nasopharyngeal carcinoma: a cohort study from 1990 to 2012. Int J Radiat Oncol Biol Phys. 2019; 105:581–90.6. Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer. 2019; 119:87–96.7. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016; 17:1509–20.8. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019; 381:1124–35.9. Tang LL, Guo R, Zhang N, Deng B, Chen L, Cheng ZB, et al. Effect of radiotherapy alone vs radiotherapy with concurrent chemoradiotherapy on survival without disease relapse in patients with low-risk nasopharyngeal carcinoma: a randomized clinical trial. JAMA. 2022; 328:728–36.10. Wang Q, Xu G, Xia Y, Zuo J, Zeng G, Xue Z, et al. Comparison of induction chemotherapy plus concurrent chemoradiotherapy and induction chemotherapy plus radiotherapy in locally advanced nasopharyngeal carcinoma. Oral Oncol. 2020; 111:104925.11. Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004; 350:2461–70.12. Vasudevan HN, Yom SS. Nasopharyngeal carcinoma and its association with Epstein-Barr virus. Hematol Oncol Clin North Am. 2021; 35:963–71.13. Qu H, Huang Y, Zhao S, Zhou Y, Lv W. Prognostic value of Epstein-Barr virus DNA level for nasopharyngeal carcinoma: a meta-analysis of 8128 cases. Eur Arch Otorhinolaryngol. 2020; 277:9–18.14. Sun XS, Chen WH, Liu SL, Liang YJ, Chen QY, Guo SS, et al. Individualized concurrent chemotherapy by pretreatment plasma Epstein-Barr viral DNA in II–III stage nasopharyngeal carcinoma: a propensity score matching analysis using a large cohort. Cancer Med. 2019; 8:4214–25.15. Lin S, Pan J, Han L, Guo Q, Hu C, Zong J, et al. Update report of nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy and hypothesis of the optimal margin. Radiother Oncol. 2014; 110:385–9.16. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003; 21:631–7.17. Wang F, Jiang C, Wang L, Yan F, Sun Q, Ye Z, et al. Influence of concurrent chemotherapy on locoregionally advanced nasopharyngeal carcinoma treated with neoadjuvant chemotherapy plus intensity-modulated radiotherapy: a retrospective matched analysis. Sci Rep. 2020; 10:2489.18. Li PJ, Lai YL, He F, Chen YY, Gu ZS, Luo W, et al. Explore the usefulness of concurrent chemotherapy in stage II nasopharyngeal carcinoma: a retrospective study. Front Pharmacol. 2021; 12:688528.19. Liu L, Fei Z, Chen M, Zhao L, Su H, Gu D, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus volumetric modulated arc therapy alone in the treatment of stage II–IVB nasopharyngeal carcinoma patients: a retrospective controlled study. Radiat Oncol. 2018; 13:148.20. Huang CL, Sun ZQ, Guo R, Liu X, Mao YP, Peng H, et al. Plasma Epstein-Barr virus DNA load after induction chemotherapy predicts outcome in locoregionally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2019; 104:355–61.21. Chen FP, Luo YS, Chen K, Li JY, Huo LQ, Shi L, et al. Circulating Epstein-Barr virus DNA level post induction chemotherapy contributes to prognostication in advanced-stage nasopharyngeal carcinoma. Eur J Cancer. 2021; 151:63–71.22. Xie X, Ren Y, Wang K, Yi B. Molecular prognostic value of circulating Epstein-Barr viral DNA in nasopharyngeal carcinoma: a meta-analysis of 27,235 cases in the endemic area of southeast Asia. Genet Test Mol Biomarkers. 2019; 23:448–59.23. Zhang AM, Fan Y, Wang XX, Xie QC, Sun JG, Chen ZT, et al. Increased treatment-related mortality with additional cisplatin-based chemotherapy in patients with nasopharyngeal carcinoma treated with standard radiotherapy. Radiother Oncol. 2012; 104:279–85.24. Li XY, Luo DH, Guo L, Mo HY, Sun R, Guo SS, et al. Deintensified chemoradiotherapy for pretreatment Epstein-Barr virus DNA-selected low-risk locoregionally advanced nasopharyngeal carcinoma: a phase II randomized noninferiority trial. J Clin Oncol. 2022; 40:1163–73.25. Liu GY, Li WZ, Wang DS, Liang H, Lv X, Ye YF, et al. Effect of capecitabine maintenance therapy plus best supportive care vs best supportive care alone on progression-free survival among patients with newly diagnosed metastatic nasopharyngeal carcinoma who had received induction chemotherapy: a phase 3 randomized clinical trial. JAMA Oncol. 2022; 8:553–61.26. Kang Y, He W, Ren C, Qiao J, Guo Q, Hu J, et al. Advances in targeted therapy mainly based on signal pathways for nasopharyngeal carcinoma. Signal Transduct Target Ther. 2020; 5:245.27. Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018; 19:1338–50.28. Adkins DR, Haddad RI. Clinical trial data of Anti-PD-1/PD-L1 therapy for recurrent or metastatic nasopharyngeal carcinoma: a review. Cancer Treat Rev. 2022; 109:102428.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiotherapy plus concurrent weekly cisplatin with or without neoadjuvant chemotherapy
- Neoadjuvant Chemotherapy for the Bulky-endophytic or Barrel-shaped Cervix
- Results of Conventional Radiotherapy in Hypopharyngeal Cancer
- Combined Modality Treatment in Nasopharyngeal Carcinoma
- Effects of Sequential Neoadjuvant Chemotherapy and Radiotherapy in Locally Advanced Non Small Cell Lung Cancer: Long Term Results