J Korean Neurosurg Soc.
2023 Nov;66(6):672-680. 10.3340/jkns.2023.0065.
Management and Outcome of Intracranial Dural Arteriovenous Fistulas That Have Caused a Hemorrhage in the Posterior Fossa : A Clinical Study
- Affiliations
-
- 1Departmenty of Neurosurgery, Bursa Yüksek İhtisas Training and Research Hospital, Bursa, Turkey
- 2Departmenty of Neurosurgery, University of Health Sciences, Ankara City Hospital, Ankara, Turkey
- 3Departmenty of Radiology, University of Health Sciences, Ankara City Hospital, Ankara,Turkey
- 4Departmenty of Neurosurgery, University of Health Sciences, Adana City Hospital, Adana, Turkey
- KMID: 2547461
- DOI: http://doi.org/10.3340/jkns.2023.0065
Abstract
Objective
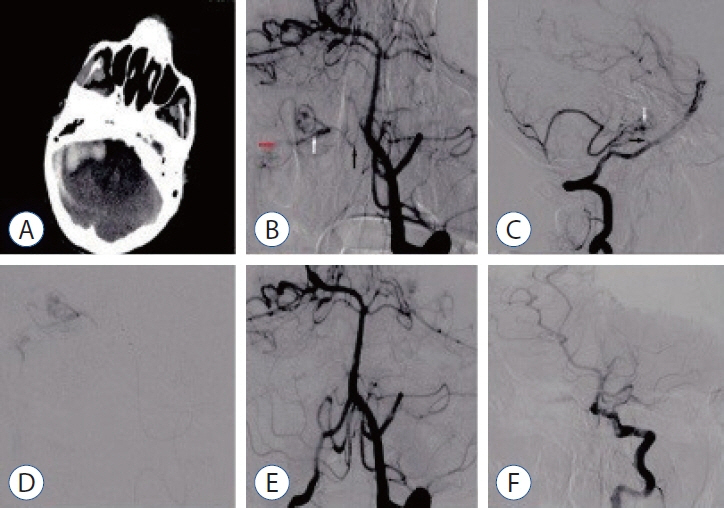
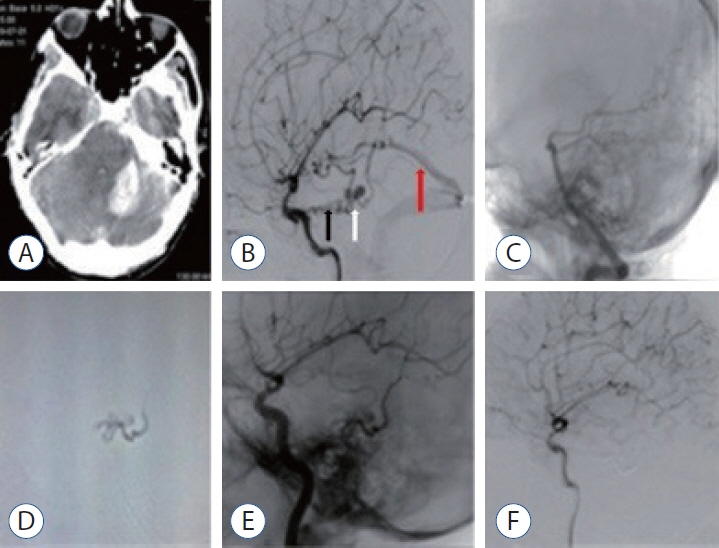
: We evaluated the diagnosis, treatment, and long-term results of patients with dural arteriovenous fistula (dAVF), which is a very rare cause of posterior fossa hemorrhage.
Methods
: This study included 15 patients who underwent endovascular, surgical, combined, or Gamma Knife treatments between 2012 and 2020. Demographics and clinical features, angiographic features, treatment modalities, and outcomes were analyzed.
Results
: The mean age of the patients was 40±17 years (range, 17–68), and 68% were men (11/15). Seven of the patients (46.6%) were in the age group of 50 years and older. While the mean Glasgow coma scale was 11.5±3.9 (range, 4–15), 46.3% presented with headache and 53.7% had stupor/coma. Four patients (26.6%) had only cerebellar hematoma and headache. All dAVFs had cortical venous drainage. In 11 patients (73.3%), the fistula was located in the tentorium and was the most common localization. Three patients (20%) had transverse and sigmoid sinus localizations, while one patient (6.7%) had dAVF located in the foramen magnum. Eighteen sessions were performed on the patients during endovascular treatment. Sixteen sessions (88.8%) were performed with the transarterial (TA) route, one session (5.5%) with the transvenous (TV) route, and one session (5.5%) with the TA+TV route. Surgery was performed in two patients (14.2%). One patient (7.1%) passed away. While there were nine patients (64.2%) with a Rankin score between 0 and 2, the total closure rate was 69.2% in the first year of control angiograms.
Conclusion
: In the differential diagnosis of posterior fossa hemorrhages, the differential diagnosis of dAVFs, which is a very rare entity, should be considered, even in the middle and elderly age groups, in patients presenting with good clinical status and pure hematoma. The treatment of such patients can be done safely and effectively in a multidisciplinary manner with a good understanding of pathological vascular anatomy and appropriate endovascular treatment approaches.
Keyword
Figure
Reference
-
References
1. Brinjikji W, Cloft HJ, Lanzino G. Clinical, angiographic, and treatment characteristics of cranial dural arteriovenous fistulas with pial arterial supply. J Neurointerv Surg. 13:331–335. 2021.
Article2. Chung SJ, Kim JS, Kim JC, Lee SK, Kwon SU, Lee MC, et al. Intracranial dural arteriovenous fistulas: analysis of 60 patients. Cerebrovasc Dis. 13:79–88. 2002.
Article3. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 194:671–680. 1995.
Article4. Daniels DJ, Vellimana AK, Zipfel GJ, Lanzino G. Intracranial hemorrhage from dural arteriovenous fistulas: clinical features and outcome. Neurosurg Focus. 34:E15. 2013.
Article5. Datar S, Rabinstein AA. Cerebellar hemorrhage. Neurol Clin. 32:993–1007. 2014.
Article6. Détraz L, Orlov K, Berestov V, Borodetsky V, Rouchaud A, de Abreu Mattos LG, et al. Posterior fossa dural arteriovenous fistulas with subarachnoid venous drainage: outcomes of endovascular treatment. AJNR Am J Neuroradiol. 40:1363–1368. 2019.
Article7. Duffau H, Lopes M, Janosevic V, Sichez JP, Faillot T, Capelle L, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg. 90:78–84. 1999.
Article8. Ghobrial GM, Marchan E, Nair AK, Dumont AS, Tjoumakaris SI, Gonzalez LF, et al. Dural arteriovenous fistulas: a review of the literature and a presentation of a single institution’s experience. World Neurosurg. 80:94–102. 2013.
Article9. Gross BA, Albuquerque FC, McDougall CG, Jankowitz BT, Jadhav AP, Jovin TG, et al. A multi-institutional analysis of the untreated course of cerebral dural arteriovenous fistulas. J Neurosurg. 129:1114–1119. 2018.
Article10. Guo WY, Pan DH, Wu HM, Chung WY, Shiau CY, Wang LW, et al. Radiosurgery as a treatment alternative for dural arteriovenous fistulas of the cavernous sinus. AJNR Am J Neuroradiol. 19:1081–1087. 1998.11. Hashiguchi A, Mimata C, Ichimura H, Morioka M, Kuratsu J. Venous aneurysm development associated with a dural arteriovenous fistula of the anterior cranial fossa with devastating hemorrhage--case report. Neurol Med Chir (Tokyo). 47:70–73. 2007.
Article12. Jabbour P, Tjoumakaris S, Chalouhi N, Randazzo C, Gonzalez LF, Dumont A, et al. Endovascular treatment of cerebral dural and pial arteriovenous fistulas. Neuroimaging Clin N Am. 23:625–636. 2013.
Article13. King WA, Martin NA. Intracerebral hemorrhage due to dural arteriovenous malformations and fistulae. Neurosurg Clin N Am. 3:577–590. 1992.
Article14. Lawton MT, Sanchez-Mejia RO, Pham D, Tan J, Halbach VV. Tentorial dural arteriovenous fistulae: operative strategies and microsurgical results for six types. Neurosurgery. 62(3 Suppl 1):110–24. discussion 124-125. 2008.15. Li C, Yang X, Li Y, Jiang C, Wu Z. Endovascular treatment of intracranial dural arteriovenous fistulas presenting with intracranial hemorrhage in 46 consecutive patients: with emphasis on transarterial embolization with onyx. Clin Neuroradiol. 26:301–308. 2016.
Article16. Mulholland CB, Kalani MYS, Albuquerque FC. Endovascular management of intracranial dural arteriovenous fistulas. Handb Clin Neurol. 143:117–123. 2017.
Article17. Nose T, Maki Y, Ono Y, Yoshizawa T, Tsuboi K. Computed tomography in hypertensive cerebellar hemorrhage (author’s transl). Neurol Surg. 9:1409–1415. 1981.18. Peto I, Abou-Al-Shaar H, White TG, Kwan K, Wagner K, Prashant GN, et al. Interdisciplinary treatment of posterior fossa dural arteriovenous fistulas. Acta Neurochir (Wien). 163:2515–2524. 2021.
Article19. Qureshi AM, Bhatia K, Kostynskyy A, Krings T. Clinical and angioarchitectural features of ruptured dural arteriovenous fistulas. World Neurosurg. 147:e476–e481. 2021.
Article20. Rutkowski MJ, Jian B, Lawton MT. Surgical management of cerebral dural arteriovenous fistulae. Handb Clin Neurol. 143:107–116. 2017.
Article21. Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 40:394–399. 2009.
Article22. Satoh K, Satomi J, Nakajima N, Matsubara S, Nagahiro S. Cerebellar hemorrhage caused by dural arteriovenous fistula: a review of five cases. J Neurosurg. 94:422–426. 2001.
Article23. Singh V, Smith WS, Lawton MT, Halbach VV, Young WL. Risk factors for hemorrhagic presentation in patients with dural arteriovenous fistulae. Neurosurgery. 62:628–635. discussion 628-635. 2008.
Article24. Sorteberg W Jr, Sorteberg A Jr, Jacobsen EA Jr, Rønning P Jr, Eide PK Jr. Intracranial hemorrhage from dural arteriovenous fistulas: symptoms, early rebleed, and acute management: a single-center 8-year experience. Neurosurgery Open. 1:okaa019. 2020.
Article25. Sundt TM Jr, Piepgras DG. The surgical approach to arteriovenous malformations of the lateral and sigmoid dural sinuses. J Neurosurg. 59:32–39. 1983.
Article26. Tong X, Wu J, Lin F, Cao Y, Zhao Y, Wang S, et al. Cerebellar arteriovenous malformations: clinical feature, risk of hemorrhage and predictors of posthemorrhage outcome. World Neurosurg. 92:206–217. 2016.
Article27. Wu Q, Zhang XS, Wang HD, Zhang QR, Wen LL, Hang CH, et al. Onyx embolization for tentorial dural arteriovenous fistula with pial arterial supply: case series and analysis of complications. World Neurosurg. 92:58–64. 2016.
Article28. Yanaka K, Meguro K, Fujita K, Narushima K, Nose T. Postoperative brainstem high intensity is correlated with poor outcomes for patients with spontaneous cerebellar hemorrhage. Neurosurgery. 45:1323–1327. discussion 1327-1328. 1999.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dural Arteriovenous Malformation on the Anterior Cranial Fossa
- Surgical Treatment of Carotid-Cavernous Fistula and Intracranial Dural Arteriovenous Malformations
- Role of surgery in management of intracranial dural arteriovenous fistulas
- Clinical Study on the Intracranial Arteriovenous Malformation
- Posterior Fossa Dural Arteriovenous Malformation: Case Report