Endocrinol Metab.
2023 Oct;38(5):557-567. 10.3803/EnM.2023.1672.
Protective Effects of Melatonin in High-Fat Diet-Induced Hepatic Steatosis via Decreased Intestinal Lipid Absorption and Hepatic Cholesterol Synthesis
- Affiliations
-
- 1Department of Internal Medicine, Kosin University College of Medicine
- 2Department of Surgery,Kosin University College of Medicine
- 3Department of Food Science and Nutrition, Pusan National University, Busan, Korea
- KMID: 2546986
- DOI: http://doi.org/10.3803/EnM.2023.1672
Abstract
- Background
The preventative effect of melatonin on the development of obesity and the progression of fatty liver under a high-fat diet (HFD) has been well elucidated through previous studies. We investigated the mechanism behind this effect regarding cholesterol biosynthesis and regulation of cholesterol levels.
Methods
Mice were divided into three groups: normal chow diet (NCD); HFD; and HFD and melatonin administration group (HFD+M). We assessed the serum lipid profile, mRNA expression levels of proteins involved in cholesterol synthesis and reabsorption in the liver and nutrient transporters in the intestines, and cytokine levels. Additionally, an in vitro experiment using HepG2 cells was performed.
Results
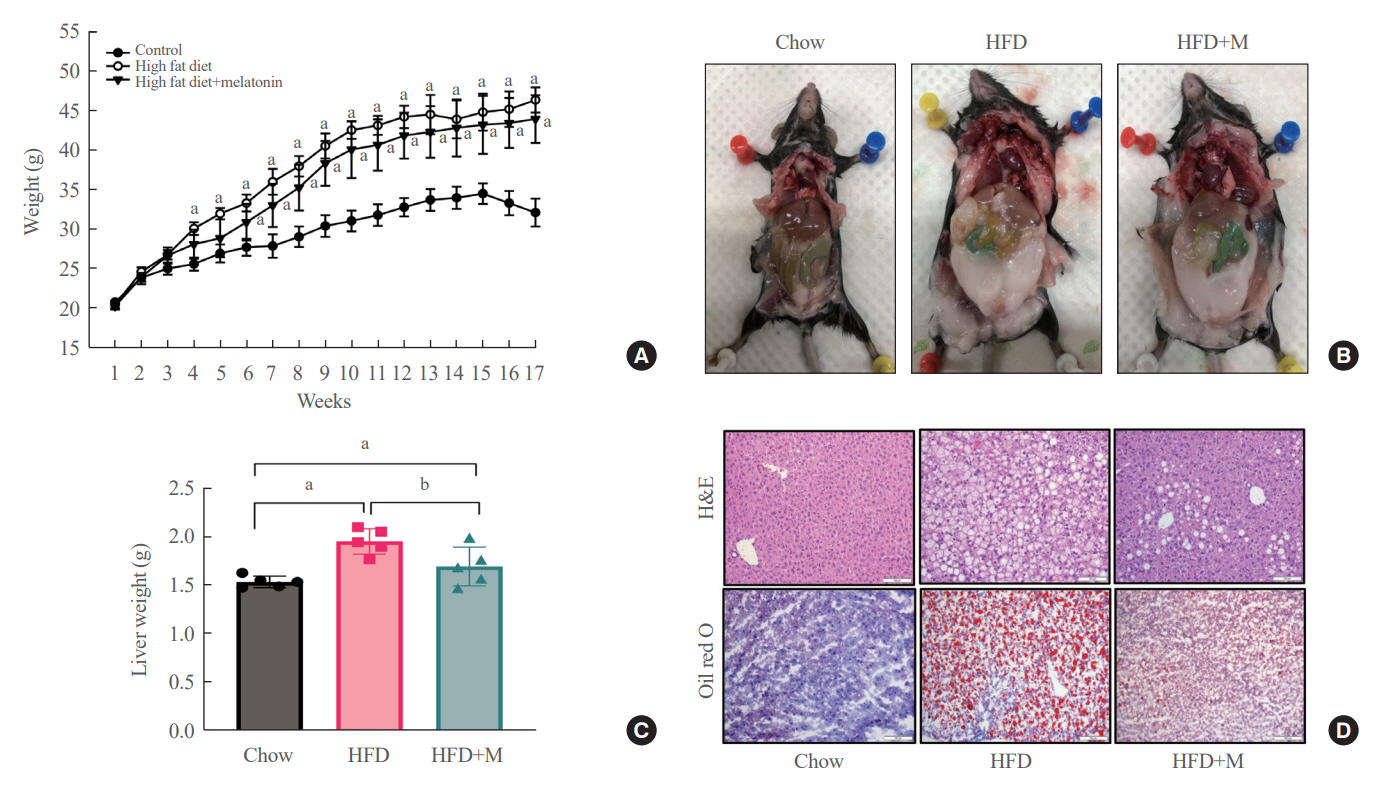
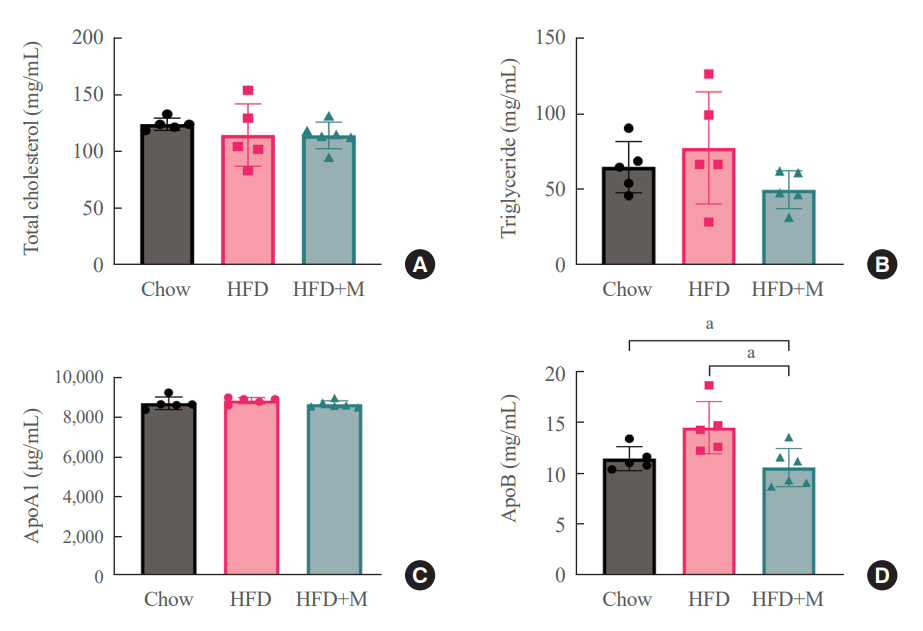
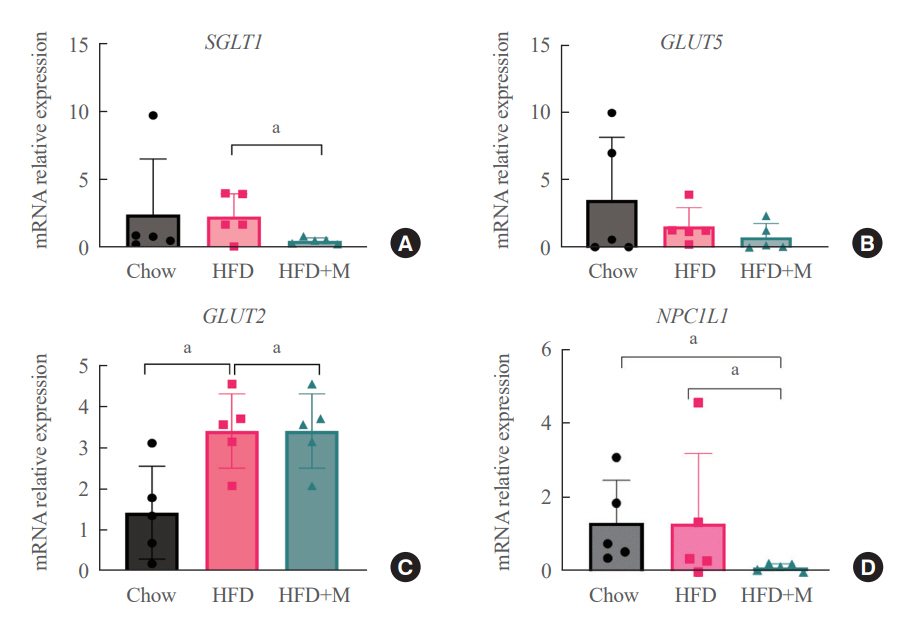
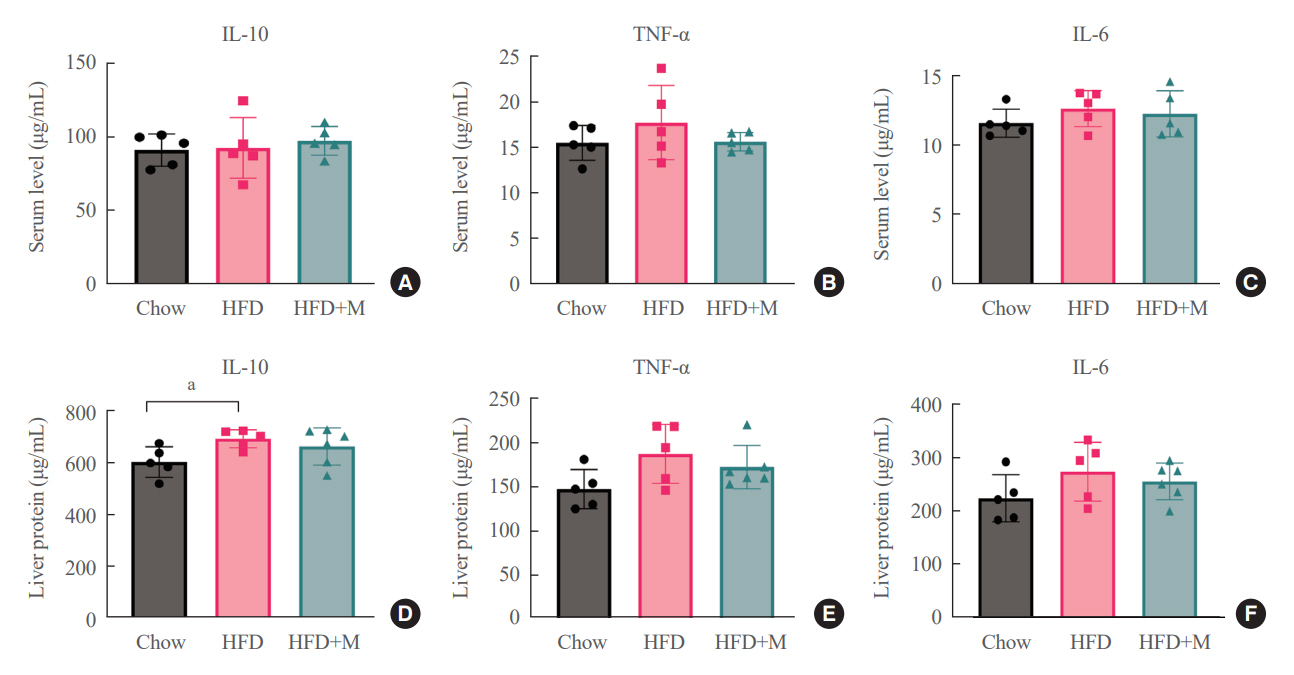
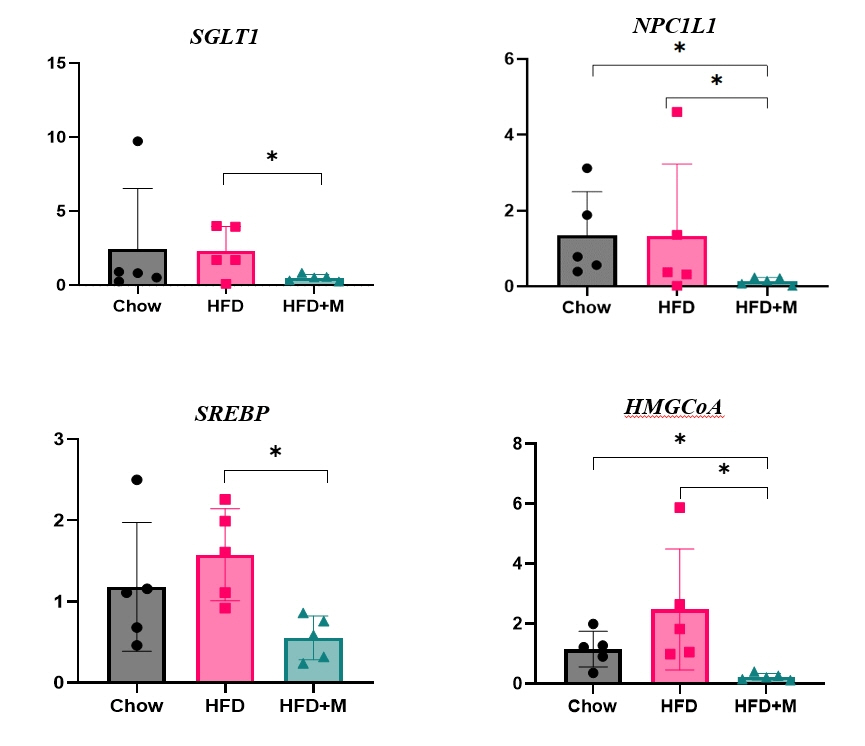
Expression of hepatic sterol regulatory element-binding protein 2 (SREBP-2), 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), and low-density lipoprotein receptor (LDLR) demonstrated that melatonin administration significantly reduces hepatic cholesterol synthesis in mice fed an HFD. Expression of intestinal sodium-glucose transporter 1 (SGLT1), glucose transporter 2 (GLUT2), GLUT5, and Niemann-pick C1-like 1 (NPC1L1) demonstrated that melatonin administration significantly reduces intestinal carbohydrate and lipid absorption in mice fed an HFD. There were no differences in local and circulatory inflammatory cytokine levels among the NCD, HFD, and HFD+M group. HepG2 cells stimulated with palmitate showed reduced levels of SREBP, LDLR, and HMGCR indicating these results are due to the direct mechanistic effect of melatonin on hepatocytes.
Conclusion
Collectively, these data indicate the mechanism behind the protective effects of melatonin from weight gain and liver steatosis under HFD is through a reduction in intestinal caloric absorption and hepatic cholesterol synthesis highlighting its potential in the treatment of obesity and fatty liver disease.
Keyword
Figure
Reference
-
1. World Health Organization. Obesity and overweight [Internet]. Geneva: WHO;2021. [cited 2023 Jul 31]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017; 390:2627–42.3. Ha KH, Kim DJ. Epidemiology of childhood obesity in Korea. Endocrinol Metab (Seoul). 2016; 31:510–8.
Article4. Rhie S, Lee S, Chae KY. Sleep patterns and school performance of Korean adolescents assessed using a Korean version of the pediatric daytime sleepiness scale. Korean J Pediatr. 2011; 54:29–35.
Article5. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010; 33:585–92.
Article6. Kim BK, Kim BS, An SY, Lee MS, Choi YJ, Han SJ, et al. Sleep duration and glycemic control in patients with diabetes mellitus: Korea National Health and Nutrition Examination Survey 2007-2010. J Korean Med Sci. 2013; 28:1334–9.
Article7. Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008; 1129:287–304.
Article8. Kohansieh M, Makaryus AN. Sleep deficiency and deprivation leading to cardiovascular disease. Int J Hypertens. 2015; 2015:615681.
Article9. Sato K, Meng F, Francis H, Wu N, Chen L, Kennedy L, et al. Melatonin and circadian rhythms in liver diseases: functional roles and potential therapies. J Pineal Res. 2020; 68:e12639.
Article10. Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006; 29:657–61.
Article11. Yeo Y, Ma SH, Park SK, Chang SH, Shin HR, Kang D, et al. A prospective cohort study on the relationship of sleep duration with all-cause and disease-specific mortality in the Korean Multi-center Cancer Cohort study. J Prev Med Public Health. 2013; 46:271–81.
Article12. Rodriguez V, Mellado C, Alvarez E, De Diego JG, Blazquez E. Effect of pinealectomy on liver insulin and glucagon receptor concentrations in the rat. J Pineal Res. 1989; 6:77–88.
Article13. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014; 56:371–81.
Article14. Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, et al. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab. 2013; 98:337–44.
Article15. Szewczyk-Golec K, Wozniak A, Reiter RJ. Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: implications for obesity. J Pineal Res. 2015; 59:277–91.
Article16. She M, Laudon M, Yin W. Melatonin receptors in diabetes: a potential new therapeutical target? Eur J Pharmacol. 2014; 744:220–3.
Article17. Xu P, Wang J, Hong F, Wang S, Jin X, Xue T, et al. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017; 62:e12399.
Article18. Duan Y, Gong K, Xu S, Zhang F, Meng X, Han J. Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics. Signal Transduct Target Ther. 2022; 7:265.
Article19. Jia L, Betters JL, Yu L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu Rev Physiol. 2011; 73:239–59.
Article20. Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020; 21:225–45.
Article21. Wang L, McFadden JW, Yang G, Zhu H, Lian H, Fu T, et al. Effect of melatonin on visceral fat deposition, lipid metabolism and hepatic lipo-metabolic gene expression in male rats. J Anim Physiol Anim Nutr (Berl). 2021; 105:787–96.
Article22. Mi Y, Tan D, He Y, Zhou X, Zhou Q, Ji S. Melatonin modulates lipid metabolism in HepG2 cells cultured in high concentrations of oleic acid: AMPK pathway activation may play an important role. Cell Biochem Biophys. 2018; 76:463–70.
Article23. Hussain SA. Effect of melatonin on cholesterol absorption in rats. J Pineal Res. 2007; 42:267–71.
Article24. Michurina SV, Kolesnikov SI, Ishchenko IY, Arkhipov SA. Light-induced functional pinealectomy: expression of MT2 receptors in liver cells of C57BL/6 mice after melatonin treatment. Bull Exp Biol Med. 2022; 173:569–74.
Article25. Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol. 2011; 17:3888–98.
Article26. Tung YT, Chiang PC, Chen YL, Chien YW. Effects of melatonin on lipid metabolism and circulating irisin in Sprague-Dawley rats with diet-induced obesity. Molecules. 2020; 25:3329.
Article27. Keskin I, Kaplan S, Kalkan S, Sutcu M, Ulkay MB, Esener OB. Evaluation of neuroprotection by melatonin against adverse effects of prenatal exposure to a nonsteroidal anti-inflammatory drug during peripheral nerve development. Int J Dev Neurosci. 2015; 41:1–7.
Article28. Onger ME, Kaplan S, Deniz OG, Altun G, Altunkaynak BZ, Balci K, et al. Possible promoting effects of melatonin, leptin and alcar on regeneration of the sciatic nerve. J Chem Neuroanat. 2017; 81:34–41.
Article29. Mirza MS. Obesity, visceral fat, and NAFLD: querying the role of adipokines in the progression of nonalcoholic fatty liver disease. ISRN Gastroenterol. 2011; 2011:592404.30. Sun H, Huang FF, Qu S. Melatonin: a potential intervention for hepatic steatosis. Lipids Health Dis. 2015; 14:75.
Article31. Prunet-Marcassus B, Desbazeille M, Bros A, Louche K, Delagrange P, Renard P, et al. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology. 2003; 144:5347–52.
Article32. Shieh JM, Wu HT, Cheng KC, Cheng JT. Melatonin ameliorates high fat diet-induced diabetes and stimulates glycogen synthesis via a PKCzeta-Akt-GSK3beta pathway in hepatic cells. J Pineal Res. 2009; 47:339–44.33. Behbodikhah J, Ahmed S, Elyasi A, Kasselman LJ, De Leon J, Glass AD, et al. Apolipoprotein B and cardiovascular disease: biomarker and potential therapeutic target. Metabolites. 2021; 11:690.
Article34. Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009; 296:E1183–94.
Article35. Mohammadi-Sartang M, Ghorbani M, Mazloom Z. Effects of melatonin supplementation on blood lipid concentrations: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018; 37(6 Pt A):1943–54.
Article36. Mathes AM. Hepatoprotective actions of melatonin: possible mediation by melatonin receptors. World J Gastroenterol. 2010; 16:6087–97.
Article37. Gomez-Lechon MJ, Donato MT, Martinez-Romero A, Jimenez N, Castell JV, O’Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007; 165:106–16.
Article38. Park JY, Kim Y, Im JA, Lee H. Oligonol suppresses lipid accumulation and improves insulin resistance in a palmitateinduced in HepG2 hepatocytes as a cellular steatosis model. BMC Complement Altern Med. 2015; 15:185.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Genistein Supplementation on Fatty Liver and Lipid Metabolism in Rats Fed High Fat Diet
- Inhibition of Serotonin Synthesis Induces Negative Hepatic Lipid Balance
- Folic acid supplementation prevents high fructose-induced non-alcoholic fatty liver disease by activating the AMPK and LKB1 signaling pathways
- Sour cherry ameliorates hepatic lipid synthesis in high-fat diet-induced obese mice via activation of adenosine monophosphate-activated protein kinase signaling
- Umbelliferone Ameliorates Hepatic Steatosis and Lipid-Induced ER Stress in High-Fat Diet-Induced Obese Mice