Korean J Transplant.
2023 Sep;37(3):145-154. 10.4285/kjt.23.0034.
Recommendations for SARS-CoV-2 testing and organ procurement from deceased donors in the Republic of Korea
- Affiliations
-
- 1Division of Infectious Diseases, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 2Division of Infectious Diseases, Department of Internal Medicine, Inje University Busan Paik Hospital, College of Medicine, Inje University, Busan, Korea
- 3Department of Pediatrics, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea
- 4Korea Organ Donation Agency, Seoul, Korea
- 5Division of Infectious Diseases, Chungnam National University Sejong Hospital, Sejong, Korea
- 6Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Division of Infectious Diseases, Department of Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 8Division of Infectious Diseases, Department of Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 9Division of Infectious Disease, Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea
- 10Division of Infectious Diseases, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea
- 11Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 12Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 13Division of Infectious Diseases, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 14Department of Infectious Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 15Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2546472
- DOI: http://doi.org/10.4285/kjt.23.0034
Abstract
- We present a summary of the evidence on testing for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and organ procurement from deceased donors and provide recommendations based on current clinical data and the guidelines from major transplant organizations. Because of the limited historical experience with coronavirus disease 2019 (COVID-19), certain recommendations in this document are based on theoretical rationales rather than clinical data. The recommendations in this manuscript may be subject to revision as subsequent clinical studies provide definitive evidence regarding COVID-19 in organ procurement.
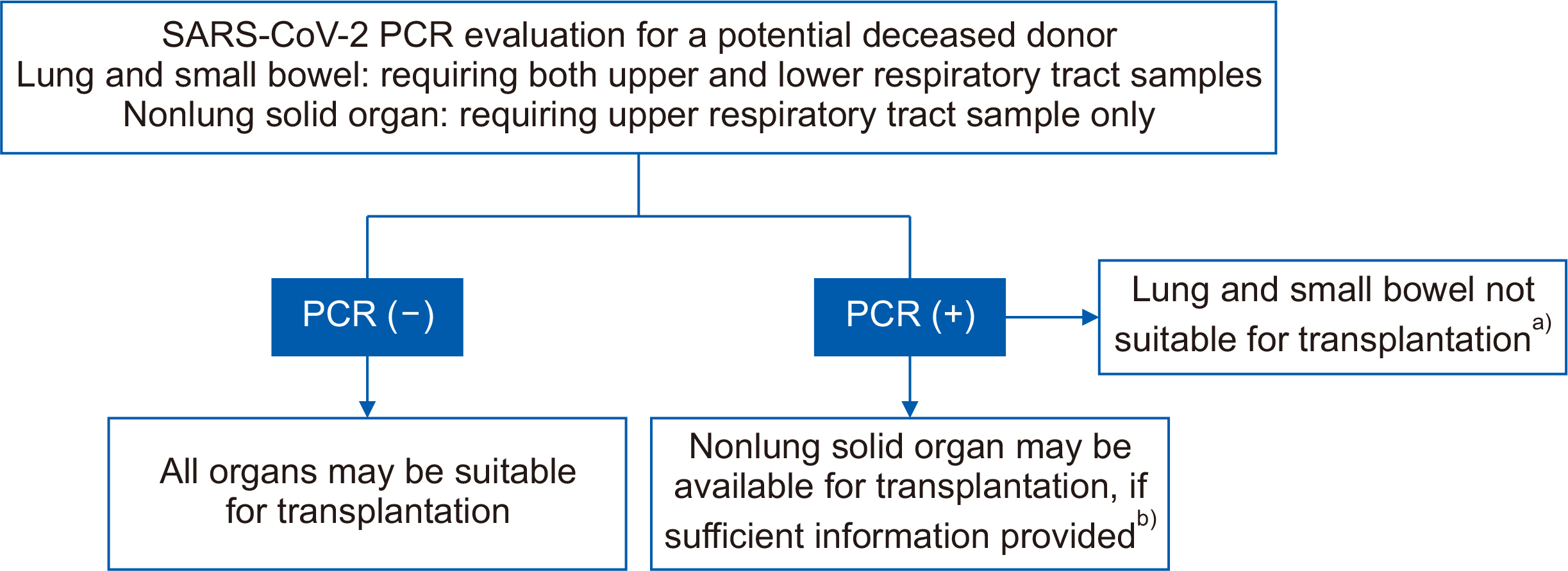
Figure
Reference
-
1. Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E. 2021; COVID-19 and solid organ transplantation: a review article. Transplantation. 105:37–55. DOI: 10.1097/TP.0000000000003523. PMID: 33148977.2. Ritschl PV, Nevermann N, Wiering L, Wu HH, Moroder P, Brandl A, et al. 2020; Solid organ transplantation programs facing lack of empiric evidence in the COVID-19 pandemic: a By-proxy Society Recommendation Consensus approach. Am J Transplant. 20:1826–36. DOI: 10.1111/ajt.15933. PMID: 32323460. PMCID: PMC7264649.3. Montiel Villalonga P, Martínez-Alpuente I, Fernández-Ruiz M, Len Ó, Bodro M, Los-Arcos I, et al. 2023; Transplantation of organs from SARS-CoV-2-positive donors: preliminary experience from Spain. Transpl Infect Dis. 25:e14008. DOI: 10.1111/tid.14008. PMID: 36659870.4. Ushiro-Lumb I, Callaghan CJ, Pettigrew GJ, Madden S, Mumford L, Currie I, et al. 2022; Transplantation of organs from SARS-CoV-2 RNA positive deceased donors: the UK experience so far. Transplantation. 106:e418–9. DOI: 10.1097/TP.0000000000004206. PMID: 35581692.5. Goldman JD, Pouch SM, Woolley AE, Booker SE, Jett CT, Fox C, et al. 2023; Transplant of organs from donors with positive SARS-CoV-2 nucleic acid testing: a report from the organ procurement and transplantation network ad hoc disease transmission advisory committee. Transpl Infect Dis. 25:e14013. DOI: 10.1111/tid.14013. PMID: 36694448.6. Organ Procurement and Transplantation Network (OPTN). 2022. Summary of current evidence and information-donor SARS-CoV-2 testing & organ recovery from donors with a history of COVID-19 [Internet]. OPTN;Available from: https://optn.transplant.hrsa.gov/media/kkhnlwah/sars-cov-2-summary-of-evidence.pdf. cited 2023 Jun 22.7. Peghin M, Grossi PA. 2022; COVID-19 positive donor for solid organ transplantation. J Hepatol. 77:1198–204. DOI: 10.1016/j.jhep.2022.06.021. PMID: 35798131. PMCID: PMC9251900.8. The Transplantation Society of Australia and New Zealand (TSANZ). 2022. Clinical guidelines for organ transplantation from deceased donors [Internet]. TSANZ;Available from: https://tsanz.com.au/storage/documents/TSANZ_Clinical_Guidelines_Version-110_Final.pdf. cited 2023 Mar 7.9. National Health Service Blood and Transplant (NHSBT). 2022. SARS-CoV-2 assessment and screening in donors and recipients [Internet]. NHSBT;Available from: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/28274/pol304.pdf. cited 2023 Jun 21.10. Weiss MJ, Hornby L, Foroutan F, Belga S, Bernier S, Bhat M, et al. 2021; Clinical practice guideline for solid organ donation and transplantation during the COVID-19 pandemic. Transplant Direct. 7:e755. DOI: 10.1097/TXD.0000000000001199. PMID: 34514110. PMCID: PMC8425831.11. Boan P, Jardine A, Pryce TM. 2022; Clinical associations of SARS-CoV-2 viral load using the first WHO international standard for SARS-CoV-2 RNA. Pathology. 54:344–50. DOI: 10.1016/j.pathol.2021.11.006. PMID: 35153071. PMCID: PMC8829673.12. Jeong YJ, Wi YM, Park H, Lee JE, Kim SH, Lee KS. 2023; Current and emerging knowledge in COVID-19. Radiology. 306:e222462. DOI: 10.1148/radiol.222462. PMID: 36625747. PMCID: PMC9846833.13. Islam N, Ebrahimzadeh S, Salameh JP, Kazi S, Fabiano N, Treanor L, et al. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst Rev. 2021; (3):CD013639. DOI: 10.1002/14651858.CD013639.pub4. PMID: 33724443. PMCID: PMC8078565.14. Free RJ, Annambhotla P, La Hoz RM, Danziger-Isakov L, Jones JM, Wang L, et al. 2022; Risk of severe acute respiratory syndrome coronavirus 2 transmission through solid organ transplantation and outcomes of coronavirus disease 2019 among recent transplant recipients. Open Forum Infect Dis. 9:ofac221. DOI: 10.1093/ofid/ofac221. PMID: 35873294. PMCID: PMC9297154.15. Kaul DR, Valesano AL, Petrie JG, Sagana R, Lyu D, Lin J, et al. 2021; Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 21:2885–9. DOI: 10.1111/ajt.16532. PMID: 33565705. PMCID: PMC8014875.16. Organ Procurement and Transplantation Network (OPTN). 2021. Notice of OPTN emergency policy change: lower respiratory SARS-CoV-2 testing for lung donors [Internet]. OPTN;Available from: https://optn.transplant.hrsa.gov/media/4576/policy_notice_lunglowerrespiratorytesting_20210426.pdf. cited 2023 Jun 21.17. Organización Nacional de Trasplantes (ONT). 2022. Infección asociada al nuevo coronavirus (COVID-19) [Internet]. ONT;Available from: https://www.ont.es/wp-content/uploads/2023/06/Recomendaciones-Donacion-y-Trasplante-frente-a-la-COVID-19-Abril-2022.pdf. cited 2023 Jun 21.18. Natori Y, Anjan S, Simkins J, Abbo L, Martin E, Garcia J, et al. 2021; Small bowel transplantation from SARS-CoV-2 respiratory PCR positive donors: is it safe? Transpl Infect Dis. 23:e13752. DOI: 10.1111/tid.13752. PMID: 34724306. PMCID: PMC8646395.19. Centers for Disease Control and Prevention (CDC). 2022. Ending isolation and precautions for people with COVID-19: interim guidance [Internet]. CDC;Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. cited 2023 Jun 21.20. Bouton TC, Atarere J, Turcinovic J, Seitz S, Sher-Jan C, Gilbert M, et al. 2023; Viral dynamics of Omicron and Delta severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants with implications for timing of release from isolation: a longitudinal cohort study. Clin Infect Dis. 76:e227–33. DOI: 10.1093/cid/ciac510. PMID: 35737948. PMCID: PMC9278204.21. Keske S, Guney-Esken G, Vatansever C, Besli Y, Kuloglu ZE, Nergiz Z, et al. 2023; Duration of infectious shedding of SARS-CoV-2 Omicron variant and its relation with symptoms. Clin Microbiol Infect. 29:221–4. DOI: 10.1016/j.cmi.2022.07.009. PMID: 35853589. PMCID: PMC9287585.22. Centers for Disease Control and Prevention (CDC). 2023. Coronavirus disease 2019 (COVID-19) 2023 case definition [Internet]. CDC;Available from: https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-covid-19/. cited 2023 Jun 21.23. Dhand A, Okumura K, Nabors C, Nishida S. 2023; Solid organ transplantation from COVID positive donors in the United States: analysis of United Network for Organ Sharing database. Transpl Infect Dis. 25:e13925. DOI: 10.1111/tid.13925. PMID: 35942924. PMCID: PMC9538265.24. Romagnoli R, Gruttadauria S, Tisone G, Maria Ettorre G, De Carlis L, Martini S, et al. 2021; Liver transplantation from active COVID-19 donors: a lifesaving opportunity worth grasping? Am J Transplant. 21:3919–25. DOI: 10.1111/ajt.16823. PMID: 34467627. PMCID: PMC8653300.25. Seeble J, Waterboer T, Hippchen T, Simon J, Kirchner M, Lim A, et al. 2022; Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): a prospective cohort study. Clin Infect Dis. 74:1191–8. DOI: 10.1093/cid/ciab611. PMID: 34223884. PMCID: PMC8394862.26. Arnold DT, Hamilton FW, Milne A, Morley AJ, Viner J, Attwood M, et al. 2021; Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 76:399–401. DOI: 10.1136/thoraxjnl-2020-216086. PMID: 33273026. PMCID: PMC7716340.27. Heesakkers H, van der Hoeven JG, Corsten S, Janssen I, Ewalds E, Simons KS, et al. 2022; Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. 327:559–65. DOI: 10.1001/jama.2022.0040. PMID: 35072716. PMCID: PMC8787680.28. Xie Y, Xu E, Bowe B, Al-Aly Z. 2022; Long-term cardiovascular outcomes of COVID-19. Nat Med. 28:583–90. DOI: 10.1038/s41591-022-01689-3. PMID: 35132265. PMCID: PMC8938267.29. American Society of Transplantation (AST). 2023. SARS-CoV-2: recommendations and guidance for organ donor testing and evaluation [Internet]. AST;Available from: https://www.myast.org/sites/default/files/Donor%20Testing%20Document1.18.23.pdf. cited 2023 Jun 21.30. Bar-On YM, Flamholz A, Phillips R, Milo R. 2020; SARS-CoV-2 (COVID-19) by the numbers. Elife. 9:e57309. DOI: 10.7554/eLife.57309. PMID: 32228860. PMCID: PMC7224694.