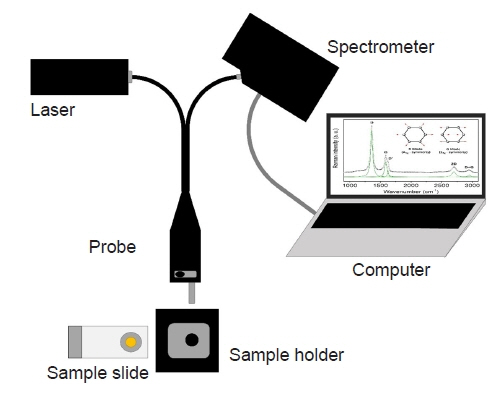
Kosin Med J.
2023 Sep;38(3):176-183. 10.7180/kmj.23.129.
Application of Raman spectroscopy in breast cancer surgery
- Affiliations
-
- 1Department of Biomedical Engineering, Ulsan National Institute of Science and Technology (UNIST), Ulsan, Korea
- 2Department of Surgery, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- KMID: 2546152
- DOI: http://doi.org/10.7180/kmj.23.129
Abstract
- The incidence of breast cancer is increasing worldwide. As cancer screening has become more widespread, the rate of early breast cancer detection has increased and treatment methods have changed. Partial mastectomy is performed more often than total mastectomy for the surgical treatment of early breast cancer, and sentinel lymph node biopsy plays an important role. A high level of accuracy is necessary for the intraoperative examination of surgical margins and sentinel lymph nodes to identify malignancies. Therefore, several examination techniques, including Raman spectroscopy, that replace or supplement the currently used frozen-section methods are being studied. Raman spectroscopy has the ability to diagnose cancer in normal tissue by providing in real time a chemical fingerprint that can be used to differentiate between cells and tissues. Numerous studies have investigated the utilization of Raman spectroscopy to identify cancer in the margins of resected tissues and sentinel lymph nodes during breast cancer surgery, showing the potential of this technique for clinical applications. This article introduces and reviews the research on Raman spectroscopy for breast cancer surgery.
Figure
Reference
-
References
1. Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020; 8:e1027–37.
Article2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article3. Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, et al. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond). 2021; 41:1183–94.
Article4. Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022; 66:15–23.
Article5. Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and surgery in early breast cancer: an overview of the randomized trials. N Engl J Med. 1995; 333:1444–56.6. Kim YS, Ryu DW, Lee CH. Comparison of survival outcomes between modified radical mastectomy and breast conserving surgery in early breast cancer patients. Kosin Med J. 2016; 31:19–29.
Article7. Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997; 15:2345–50.
Article8. Elshanbary AA, Awad AA, Abdelsalam A, Ibrahim IH, Abdel-Aziz W, Darwish YB, et al. The diagnostic accuracy of intraoperative frozen section biopsy for diagnosis of sentinel lymph node metastasis in breast cancer patients: a meta-analysis. Environ Sci Pollut Res Int. 2022; 29:47931–41.
Article9. Holck S, Galatius H, Engel U, Wagner F, Hoffmann J. False-negative frozen section of sentinel lymph node biopsy for breast cancer. Breast. 2004; 13:42–8.
Article10. McLaughlin SA, Ochoa-Frongia LM, Patil SM, Cody HS 3rd, Sclafani LM. Influence of frozen-section analysis of sentinel lymph node and lumpectomy margin status on reoperation rates in patients undergoing breast-conservation therapy. J Am Coll Surg. 2008; 206:76–82.
Article11. Aziz D, Rawlinson E, Narod SA, Sun P, Lickley HL, McCready DR, et al. The role of reexcision for positive margins in optimizing local disease control after breast-conserving surgery for cancer. Breast J. 2006; 12:331–7.
Article12. Jacobson AF, Asad J, Boolbol SK, Osborne MP, Boachie-Adjei K, Feldman SM. Do additional shaved margins at the time of lumpectomy eliminate the need for re-excision? Am J Surg. 2008; 196:556–8.
Article13. Wilke LG, Brown JQ, Bydlon TM, Kennedy SA, Richards LM, Junker MK, et al. Rapid noninvasive optical imaging of tissue composition in breast tumor margins. Am J Surg. 2009; 198:566–74.
Article14. Kennedy S, Geradts J, Bydlon T, Brown JQ, Gallagher J, Junker M, et al. Optical breast cancer margin assessment: an observational study of the effects of tissue heterogeneity on optical contrast. Breast Cancer Res. 2010; 12:R91.
Article15. Tellier F, Ravelo R, Simon H, Chabrier R, Steibel J, Poulet P. Sentinel lymph node detection by an optical method using scattered photons. Biomed Opt Express. 2010; 1:902–10.
Article16. Lu Y, Zhao Y, Zhu Y, Xu X, Yin J. In situ research and diagnosis of breast cancer by using HOF-ATR-FTIR spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2020; 235:118178.
Article17. Phipps JE, Gorpas D, Unger J, Darrow M, Bold RJ, Marcu L. Automated detection of breast cancer in resected specimens with fluorescence lifetime imaging. Phys Med Biol. 2017; 63:015003.
Article18. Balasundaram G, Goh Y, Moothanchery M, Attia A, Lim HQ, Burton NC, et al. Optoacoustic characterization of breast conserving surgery specimens: a pilot study. Photoacoustics. 2020; 19:100164.19. Butler HJ, Ashton L, Bird B, Cinque G, Curtis K, Dorney J, et al. Using Raman spectroscopy to characterize biological materials. Nat Protoc. 2016; 11:664–87.
Article20. Bao Z, Zhang Y, Tan Z, Yin X, Di W, Ye J. Gap-enhanced Raman tags for high-contrast sentinel lymph node imaging. Biomaterials. 2018; 163:105–15.
Article21. Deng B, Wang Y, Wu Y, Yin W, Lu J, Ye J. Raman nanotags-guided intraoperative sentinel lymph nodes precise location with minimal invasion. Adv Sci (Weinh). 2022; 9:e2102405.
Article22. Som D, Tak M, Setia M, Patil A, Sengupta A, Chilakapati CM, et al. A grid matrix-based Raman spectroscopic method to characterize different cell milieu in biopsied axillary sentinel lymph nodes of breast cancer patients. Lasers Med Sci. 2016; 31:95–111.
Article23. Zuniga WC, Jones V, Anderson SM, Echevarria A, Miller NL, Stashko C, et al. Raman spectroscopy for rapid evaluation of surgical margins during breast cancer lumpectomy. Sci Rep. 2019; 9:14639.
Article24. Keller MD, Vargis E, de Matos Granja N, Wilson RH, Mycek MA, Kelley MC, et al. Development of a spatially offset Raman spectroscopy probe for breast tumor surgical margin evaluation. J Biomed Opt. 2011; 16:077006.
Article25. Vankeirsbilck T, Vercauteren A, Baeyens W, Van der Weken G, Verpoort F, Vergote G, et al. Applications of Raman spectroscopy in pharmaceutical analysis. TrAC Trends Anal Chem. 2002; 21:869–77.
Article26. Eberhardt K, Stiebing C, Matthaus C, Schmitt M, Popp J. Advantages and limitations of Raman spectroscopy for molecular diagnostics: an update. Expert Rev Mol Diagn. 2015; 15:773–87.
Article27. Zhou J, Pan W, Qi W, Cao X, Cheng Z, Feng Y. Ultrafast Raman fiber laser: a review and prospect. PhotoniX. 2022; 3:18.
Article28. Ye J, Ma X, Zhang Y, Xu J, Zhang H, Yao T, et al. From spectral broadening to recompression: dynamics of incoherent optical waves propagating in the fiber. PhotoniX. 2021; 2:15.
Article29. Raman CV, Krishnan KS. A new type of secondary radiation. Nature. 1928; 121:501–2.
Article30. Ember KJ, Hoeve MA, McAughtrie SL, Bergholt MS, Dwyer BJ, Stevens MM, et al. Raman spectroscopy and regenerative medicine: a review. NPJ Regen Med. 2017; 2:12.
Article31. Choo-Smith LP, Edwards HG, Endtz HP, Kros JM, Heule F, Barr H, et al. Medical applications of Raman spectroscopy: from proof of principle to clinical implementation. Biopolymers. 2002; 67:1–9.
Article32. Kong K, Kendall C, Stone N, Notingher I. Raman spectroscopy for medical diagnostics: from in-vitro biofluid assays to in-vivo cancer detection. Adv Drug Deliv Rev. 2015; 89:121–34.33. Hanlon EB, Manoharan R, Koo TW, Shafer KE, Motz JT, Fitzmaurice M, et al. Prospects for in vivo Raman spectroscopy. Phys Med Biol. 2000; 45:R1–59.34. Kallaway C, Almond LM, Barr H, Wood J, Hutchings J, Kendall C, et al. Advances in the clinical application of Raman spectroscopy for cancer diagnostics. Photodiagnosis Photodyn Ther. 2013; 10:207–19.
Article35. Das RS, Agrawal YK. Raman spectroscopy: recent advancements, techniques and applications. Vib Spectrosc. 2011; 57:163–76.
Article36. Vaskova H. A powerful tool for material identification: Raman spectroscopy. Int J Math Model Methods Appl Sci. 2011; 5:1205–12.37. Jones RR, Hooper DC, Zhang L, Wolverson D, Valev VK. Raman techniques: fundamentals and frontiers. Nanoscale Res Lett. 2019; 14:231.
Article38. Shipp DW, Sinjab F, Notingher I. Raman spectroscopy: techniques and applications in the life sciences. Adv Opt Photonics. 2017; 9:315–428.
Article39. Bagheri M, Komsa HP. High-throughput computation of Raman spectra from first principles. Sci Data. 2023; 10:80.
Article40. El Mendili Y, Vaitkus A, Merkys A, Grazulis S, Chateigner D, Mathevet F, et al. Raman Open Database: first interconnected Raman-X-ray diffraction open-access resource for material identification. J Appl Crystallogr. 2019; 52(Pt 3):618–25.
Article41. Grazulis S, Chateigner D, Downs RT, Yokochi AF, Quiros M, Lutterotti L, et al. Crystallography Open Database: an open-access collection of crystal structures. J Appl Crystallogr. 2009; 42(Pt 4):726–9.42. Grazulis S, Daskevic A, Merkys A, Chateigner D, Lutterotti L, Quiros M, et al. Crystallography Open Database (COD): an open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012; 40(Database issue):D420–7.43. Huang L, Sun H, Sun L, Shi K, Chen Y, Ren X, et al. Rapid, label-free histopathological diagnosis of liver cancer based on Raman spectroscopy and deep learning. Nat Commun. 2023; 14:48.
Article44. Fujita K, Smith NI. Label-free molecular imaging of living cells. Mol Cells. 2008; 26:530–5.45. Xu J, Yu T, Zois CE, Cheng JX, Tang Y, Harris AL, et al. Unveiling cancer metabolism through spontaneous and coherent Raman spectroscopy and stable isotope probing. Cancers (Basel). 2021; 13:1718.
Article46. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–41.
Article47. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002; 347:1227–32.
Article48. Obedian E, Haffty BG. Negative margin status improves local control in conservatively managed breast cancer patients. Cancer J Sci Am. 2000; 6:28–33.49. Yang SI, Lee SH. Clinical outcome of positive margin of postgastrectomy with adenocarcinoma of stomach. Kosin Med J. 2012; 27:31–6.
Article50. Farouk O, Senbel A, Shetiwy M, Attia E, Abdallah A, El-Damshety O, et al. The effectiveness of intraoperative frozen section analysis of safety margins in breast conserving surgery and the role of surgeon in decreasing the rate of positive margins. Surg Sci. 2017; 8:499–509.51. Nowikiewicz T, Srutek E, Glowacka-Mrotek I, Tarkowska M, Zyromska A, Zegarski W. Clinical outcomes of an intraoperative surgical margin assessment using the fresh frozen section method in patients with invasive breast cancer undergoing breast-conserving surgery: a single center analysis. Sci Rep. 2019; 9:13441.52. Noguchi M, Minami M, Earashi M, Taniya T, Miyazaki I, Mizukami Y, et al. Intraoperative histologic assessment of surgical margins and lymph node metastasis in breast-conserving surgery. J Surg Oncol. 1995; 60:185–90.
Article53. Garcia MT, Mota BS, Cardoso N, Martimbianco AL, Ricci MD, Carvalho FM, et al. Accuracy of frozen section in intraoperative margin assessment for breast-conserving surgery: a systematic review and meta-analysis. PLoS One. 2021; 16:e0248768.
Article54. Schrenk P, Wayand W. Sentinel-node biopsy in axillary lymph-node staging for patients with multicentric breast cancer. Lancet. 2001; 357:122.
Article55. Morgan A, Howisey RL, Aldape HC, Patton RG, Rowbotham RK, Schmidt EK, et al. Initial experience in a community hospital with sentinel lymph node mapping and biopsy for evaluation of axillary lymph node status in palpable invasive breast cancer. J Surg Oncol. 1999; 72:24–30.
Article56. Weiser MR, Montgomery LL, Susnik B, Tan LK, Borgen PI, Cody HS. Is routine intraoperative frozen-section examination of sentinel lymph nodes in breast cancer worthwhile? Ann Surg Oncol. 2000; 7:651–5.
Article57. McCahill LE, Single RM, Aiello Bowles EJ, Feigelson HS, James TA, Barney T, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012; 307:467–75.
Article58. Jorns JM, Daignault S, Sabel MS, Wu AJ. Is intraoperative frozen section analysis of reexcision specimens of value in preventing reoperation in breast-conserving therapy? Am J Clin Pathol. 2014; 142:601–8.
Article59. Jorns JM, Visscher D, Sabel M, Breslin T, Healy P, Daignaut S, et al. Intraoperative frozen section analysis of margins in breast conserving surgery significantly decreases reoperative rates: one-year experience at an ambulatory surgical center. Am J Clin Pathol. 2012; 138:657–69.60. Lessells AM, Simpson JG. A retrospective analysis of the accuracy of immediate frozen section diagnosis in surgical pathology. Br J Surg. 1976; 63:327–9.
Article61. Haka AS, Volynskaya Z, Gardecki JA, Nazemi J, Lyons J, Hicks D, et al. In vivo margin assessment during partial mastectomy breast surgery using Raman spectroscopy. Cancer Res. 2006; 66:3317–22.62. Haka AS, Volynskaya Z, Gardecki JA, Nazemi J, Shenk R, Wang N, et al. Diagnosing breast cancer using Raman spectroscopy: prospective analysis. J Biomed Opt. 2009; 14:054023.
Article63. Zhang H, Wang X, Ding R, Shen L, Gao P, Xu H, et al. Characterization and imaging of surgical specimens of invasive breast cancer and normal breast tissues with the application of Raman spectral mapping: a feasibility study and comparison with randomized single-point detection method. Oncol Lett. 2020; 20:2969–76.
Article64. Kong K, Zaabar F, Rakha E, Ellis I, Koloydenko A, Notingher I. Towards intra-operative diagnosis of tumours during breast conserving surgery by selective-sampling Raman micro-spectroscopy. Phys Med Biol. 2014; 59:6141–52.
Article65. Zhang B, Zhang Z, Gao B, Zhang F, Tian L, Zeng H, et al. Raman microspectroscopy based TNM staging and grading of breast cancer. Spectrochim Acta A Mol Biomol Spectrosc. 2023; 285:121937.
Article66. Kong K, Rowlands CJ, Varma S, Perkins W, Leach IH, Koloydenko AA, et al. Diagnosis of tumors during tissue-conserving surgery with integrated autofluorescence and Raman scattering microscopy. Proc Natl Acad Sci U S A. 2013; 110:15189–94.
Article67. Horsnell JD, Kendall C, Stone N. Towards the intra-operative use of Raman spectroscopy in breast cancer-overcoming the effects of theatre lighting. Lasers Med Sci. 2016; 31:1143–9.
Article68. Hanna K, Krzoska E, Shaaban AM, Muirhead D, Abu-Eid R, Speirs V. Raman spectroscopy: current applications in breast cancer diagnosis, challenges and future prospects. Br J Cancer. 2022; 126:1125–39.
Article69. Smith J, Kendall C, Sammon A, Christie-Brown J, Stone N. Raman spectral mapping in the assessment of axillary lymph nodes in breast cancer. Technol Cancer Res Treat. 2003; 2:327–32.
Article70. Horsnell JD, Smith JA, Sattlecker M, Sammon A, Christie-Brown J, Kendall C, et al. Raman spectroscopy: a potential new method for the intra-operative assessment of axillary lymph nodes. Surgeon. 2012; 10:123–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefullness of Raman Spectroscopy in Differentiation between Cancer and Adjacent Normal Tissue of the Larynx
- Analysis of Normal and Cancer Tissue in the Stomach Using Raman Spectroscopy
- Nano-biomarker-based surface-enhanced Raman spectroscopy for noninvasive discrimination of kidney transplant rejection types
- Surgery of Breast Cancer during the Last 5 Years: More Sophisticated and Specialized?
- Raman Spectroscopy for the Endoscopic Diagnosis of Esophageal, Gastric, and Colonic Diseases