Korean J Physiol Pharmacol.
2023 Sep;27(5):471-479. 10.4196/kjpp.2023.27.5.471.
Dendritic cells resist to disulfiram-induced cytotoxicity, but reduced interleukin-12/23(p40) production
- Affiliations
-
- 1College of Veterinary Medicine, Jeju National University, Jeju 63243, Korea
- 2Veterinary Medical Research Institute, Jeju National University, Jeju 63243, Korea
- KMID: 2545537
- DOI: http://doi.org/10.4196/kjpp.2023.27.5.471
Abstract
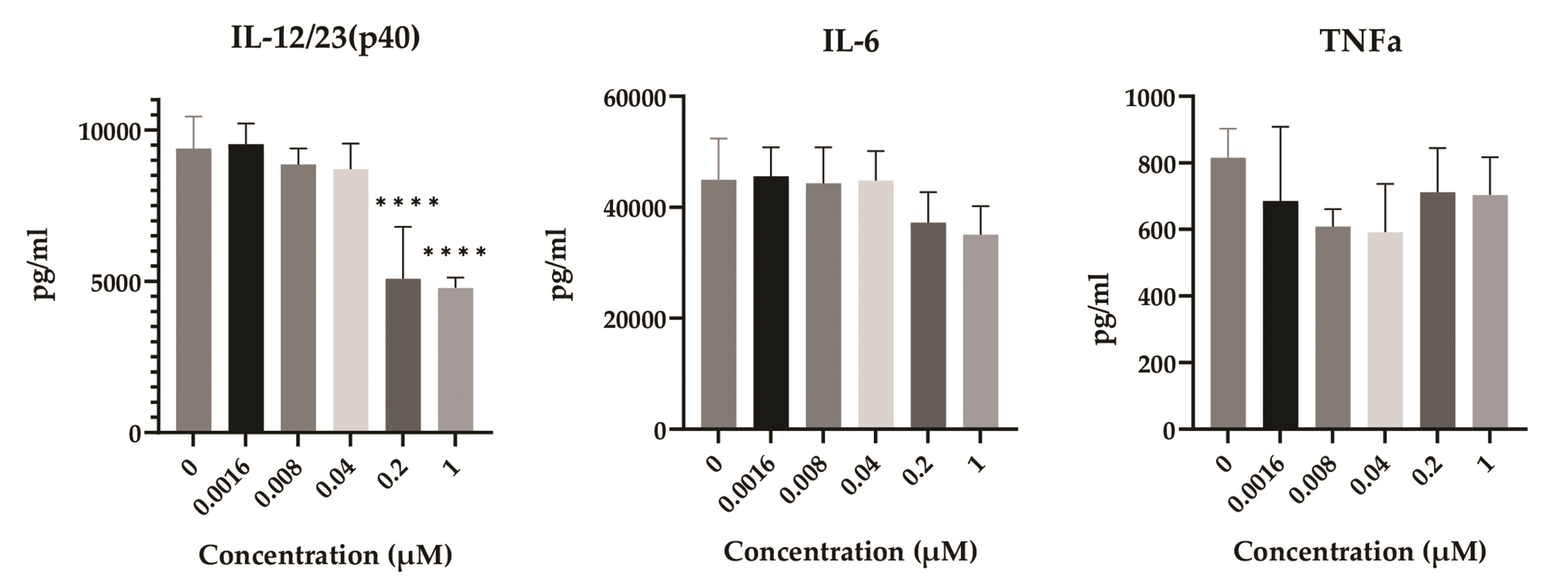
- Disulfiram (DSF), a medication for alcoholism, has recently been used as a repurposing drug owing to its anticancer effects. Despite the crucial role of dendritic cells (DCs) in immune homeostasis and cancer therapy, the effects of DSF on the survival and function of DCs have not yet been studied. Therefore, we treated bone marrow-derived DCs with DSF and lipopolysaccharide (LPS) and performed various analyses. DCs are resistant to DSF and less cytotoxic than bone marrow cells and spleen cells. The viability and metabolic activity of DCs hardly decreased after treatment with DSF in the absence or presence of LPS. DSF did not alter the expression of surface markers (MHC II, CD86, CD40, and CD54), antigen uptake capability, or the antigen-presenting ability of LPS-treated DCs. DSF decreased the production of interleukin (IL)-12/23 (p40), but not IL-6 or tumor necrosis factor-α, in LPS-treated DCs. We considered the granulocyte-macrophage colony-stimulating factor (GM-CSF) as a factor to make DCs resistant to DSF-induced cytotoxicity. The resistance of DCs to DSF decreased when GM-CSF was not given or its signaling was inhibited. Also, GM-CSF upregulated the expression of a transcription factor XBP-1 which is essential for DCs’ survival. This study demonstrated for the first time that DSF did not alter the function of DCs, had low cytotoxicity, and induced differential cytokine production.
Figure
Reference
-
1. Johansson B. 1992; A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr Scand Suppl. 369:15–26. DOI: 10.1111/j.1600-0447.1992.tb03310.x. PMID: 1471547.
Article2. Lu C, Li X, Ren Y, Zhang X. 2021; Disulfiram: a novel repurposed drug for cancer therapy. Cancer Chemother Pharmacol. 87:159–172. DOI: 10.1007/s00280-020-04216-8. PMID: 33426580.
Article3. McMahon A, Chen W, Li F. 2020; Old wine in new bottles: advanced drug delivery systems for disulfiram-based cancer therapy. J Control Release. 319:352–359. DOI: 10.1016/j.jconrel.2020.01.001. PMID: 31911155.
Article4. Skrott Z, Majera D, Gursky J, Buchtova T, Hajduch M, Mistrik M, Bartek J. 2019; Disulfiram's anti-cancer activity reflects targeting NPL4, not inhibition of aldehyde dehydrogenase. Oncogene. 38:6711–6722. DOI: 10.1038/s41388-019-0915-2. PMID: 31391554.
Article5. Terashima Y, Toda E, Itakura M, Otsuji M, Yoshinaga S, Okumura K, Shand FHW, Komohara Y, Takeda M, Kokubo K, Chen MC, Yokoi S, Rokutan H, Kofuku Y, Ohnishi K, Ohira M, Iizasa T, Nakano H, Okabe T, Kojima H, et al. 2020; Targeting FROUNT with disulfiram suppresses macrophage accumulation and its tumor-promoting properties. Nat Commun. 11:609. DOI: 10.1038/s41467-020-14338-5. PMID: 32001710. PMCID: PMC6992764. PMID: a4d75cebbb8f47a985d666129063bb6d.
Article6. Yip NC, Fombon IS, Liu P, Brown S, Kannappan V, Armesilla AL, Xu B, Cassidy J, Darling JL, Wang W. 2011; Disulfiram modulated ROS-MAPK and NFκB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer. 104:1564–1574. DOI: 10.1038/bjc.2011.126. PMID: 21487404. PMCID: PMC3101904.
Article7. Zha J, Chen F, Dong H, Shi P, Yao Y, Zhang Y, Li R, Wang S, Li P, Wang W, Xu B. 2014; Disulfiram targeting lymphoid malignant cell lines via ROS-JNK activation as well as Nrf2 and NF-kB pathway inhibition. J Transl Med. 12:163. DOI: 10.1186/1479-5876-12-163. PMID: 24915933. PMCID: PMC4075939.
Article8. Kannappan V, Ali M, Small B, Rajendran G, Elzhenni S, Taj H, Wang W, Dou QP. 2021; Recent advances in repurposing disulfiram and disulfiram derivatives as copper-dependent anticancer agents. Front Mol Biosci. 8:741316. DOI: 10.3389/fmolb.2021.741316. PMID: 34604310. PMCID: PMC8484884. PMID: fcaf5f20e5dc494488e3a449e9c91401.
Article9. Steinman RM. 2012; Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 30:1–22. DOI: 10.1146/annurev-immunol-100311-102839. PMID: 22136168.
Article10. Qian C, Cao X. 2018; Dendritic cells in the regulation of immunity and inflammation. Semin Immunol. 35:3–11. DOI: 10.1016/j.smim.2017.12.002. PMID: 29242034.
Article11. Audiger C, Rahman MJ, Yun TJ, Tarbell KV, Lesage S. 2017; The importance of dendritic cells in maintaining immune tolerance. J Immunol. 198:2223–2231. DOI: 10.4049/jimmunol.1601629. PMID: 28264998. PMCID: PMC5343761.
Article12. Kim CW, Kim KD, Lee HK. 2021; The role of dendritic cells in tumor microenvironments and their uses as therapeutic targets. BMB Rep. 54:31–43. DOI: 10.5483/BMBRep.2021.54.1.224. PMID: 33298246. PMCID: PMC7851442.
Article13. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. 2020; Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 20:7–24. DOI: 10.1038/s41577-019-0210-z. PMID: 31467405.
Article14. Fasehee H, Zarrinrad G, Tavangar SM, Ghaffari SH, Faghihi S. 2016; The inhibitory effect of disulfiram encapsulated PLGA NPs on tumor growth: different administration routes. Mater Sci Eng C Mater Biol Appl. 63:587–595. DOI: 10.1016/j.msec.2016.03.023. PMID: 27040254.
Article15. Masten BJ, Yates JL, Pollard Koga AM, Lipscomb MF. 1997; Characterization of accessory molecules in murine lung dendritic cell function: roles for CD80, CD86, CD54, and CD40L. Am J Respir Cell Mol Biol. 16:335–342. DOI: 10.1165/ajrcmb.16.3.9070619. PMID: 9070619.
Article16. Blanco P, Palucka AK, Pascual V, Banchereau J. 2008; Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 19:41–52. DOI: 10.1016/j.cytogfr.2007.10.004. PMID: 18258476. PMCID: PMC2413068.
Article17. Neurath MF. 2007; IL-23: a master regulator in Crohn disease. Nat Med. 13:26–28. DOI: 10.1038/nm0107-26. PMID: 17206128.
Article18. Iwakura Y, Ishigame H. 2006; The IL-23/IL-17 axis in inflammation. J Clin Invest. 116:1218–1222. DOI: 10.1172/JCI28508. PMID: 16670765. PMCID: PMC1451213.
Article19. Opferman JT. 2008; Apoptosis in the development of the immune system. Cell Death Differ. 15:234–242. DOI: 10.1038/sj.cdd.4402182. PMID: 17571082.
Article20. Baird AM, Gerstein RM, Berg LJ. 1999; The role of cytokine receptor signaling in lymphocyte development. Curr Opin Immunol. 11:157–166. DOI: 10.1016/S0952-7915(99)80027-2. PMID: 10322150.
Article21. Zhang N, Hartig H, Dzhagalov I, Draper D, He YW. 2005; The role of apoptosis in the development and function of T lymphocytes. Cell Res. 15:749–769. DOI: 10.1038/sj.cr.7290345. PMID: 16246265.
Article22. Hildeman D, Jorgensen T, Kappler J, Marrack P. 2007; Apoptosis and the homeostatic control of immune responses. Curr Opin Immunol. 19:516–521. DOI: 10.1016/j.coi.2007.05.005. PMID: 17644328. PMCID: PMC4127626.
Article23. Sallusto F, Lanzavecchia A. 1994; Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 179:1109–1118. DOI: 10.1084/jem.179.4.1109. PMID: 8145033. PMCID: PMC2191432.
Article24. Chen M, Wang J. 2010; Programmed cell death of dendritic cells in immune regulation. Immunol Rev. 236:11–27. DOI: 10.1111/j.1600-065X.2010.00916.x. PMID: 20636805. PMCID: PMC3282617.
Article25. Fujita Y, Matsuoka N, Temmoku J, Furuya-Yashiro M, Asano T, Sato S, Matsumoto H, Watanabe H, Kozuru H, Yatsuhashi H, Kawakami A, Migita K. 2020; JAK inhibitors impair GM-CSF-mediated signaling in innate immune cells. BMC Immunol. 21:35. DOI: 10.1186/s12865-020-00365-w. PMID: 32539713. PMCID: PMC7296727. PMID: 2122a39e70894270b93fd834c7961c85.
Article26. Iwakoshi NN, Pypaert M, Glimcher LH. 2007; The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med. 204:2267–2275. DOI: 10.1084/jem.20070525. PMID: 17875675. PMCID: PMC2118458.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- IL-12 p40-Expressing Immune Cells Revealed by Cytokine Reporter Mouse System
- Mycobacterium tuberculosis ESAT6 Drives the Activation and Maturation of Bone Marrow-Derived Dendritic Cells via TLR4-Mediated Signaling
- Induction of IL-12 Experession in Bone Marrow-derived Mouse Dendritic Cells
- The Role of Interleukin-12 in Dendritic Cells
- BCG-Induced Dendritic Cell Responses and Suppression of Interleukin-5 Production from T Cells in Atopic Asthmatics