Diabetes Metab J.
2023 Jul;47(4):500-513. 10.4093/dmj.2022.0110.
Beneficial Effects of a Curcumin Derivative and Transforming Growth Factor-β Receptor I Inhibitor Combination on Nonalcoholic Steatohepatitis
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
- 2Research Institute of Metabolism and Inflammation, Yonsei University Wonju College of Medicine, Wonju, Korea
- 3Department of Pharmacy, College of Pharmacy, Ewha Womans University, Seoul, Korea
- 4Department of Basic Science, Yonsei University Wonju College of Medicine, Wonju, Korea
- KMID: 2544729
- DOI: http://doi.org/10.4093/dmj.2022.0110
Abstract
- Background
Curcumin 2005-8 (Cur5-8), a derivative of curcumin, improves fatty liver disease via AMP-activated protein kinase activation and autophagy regulation. EW-7197 (vactosertib) is a small molecule inhibitor of transforming growth factor β (TGF-β) receptor I and may scavenge reactive oxygen species and ameliorate fibrosis through the SMAD2/3 canonical pathway. This study aimed to determine whether co-administering these two drugs having different mechanisms is beneficial.
Methods
Hepatocellular fibrosis was induced in mouse hepatocytes (alpha mouse liver 12 [AML12]) and human hepatic stellate cells (LX-2) using TGF-β (2 ng/mL). The cells were then treated with Cur5-8 (1 μM), EW-7197 (0.5 μM), or both. In animal experiments were also conducted during which, methionine-choline deficient diet, Cur5-8 (100 mg/kg), and EW-7197 (20 mg/kg) were administered orally to 8-week-old C57BL/6J mice for 6 weeks.
Results
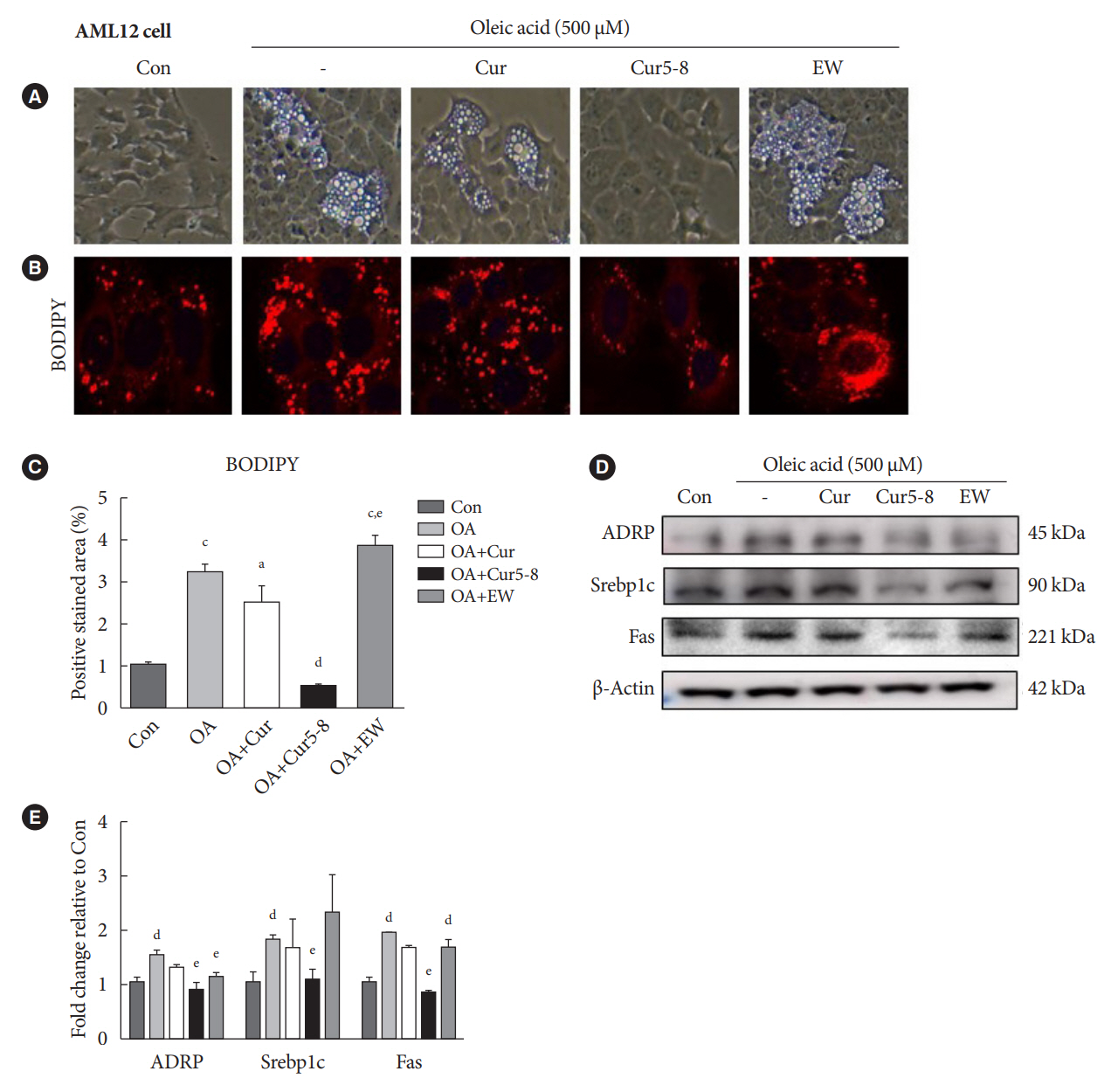
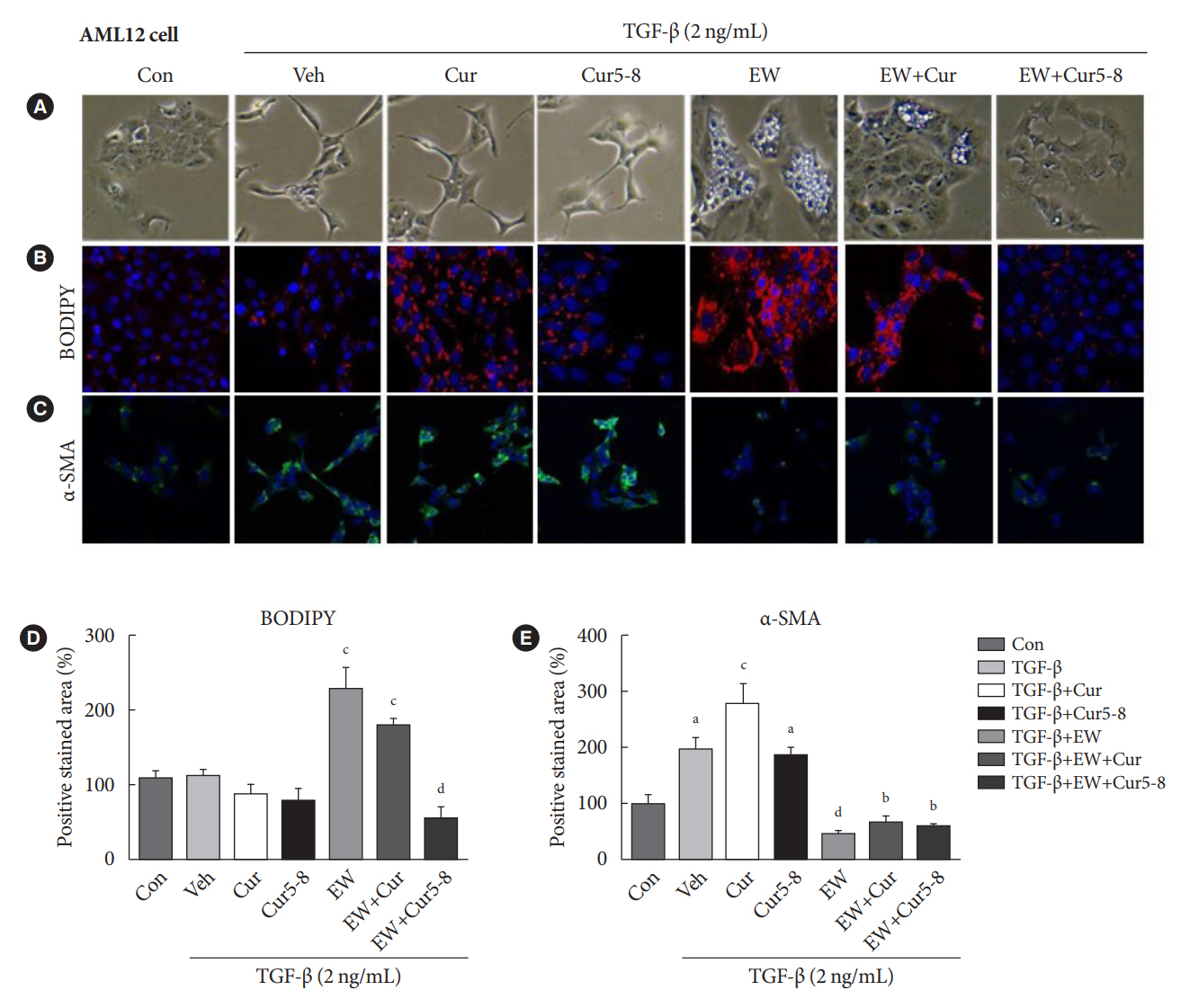
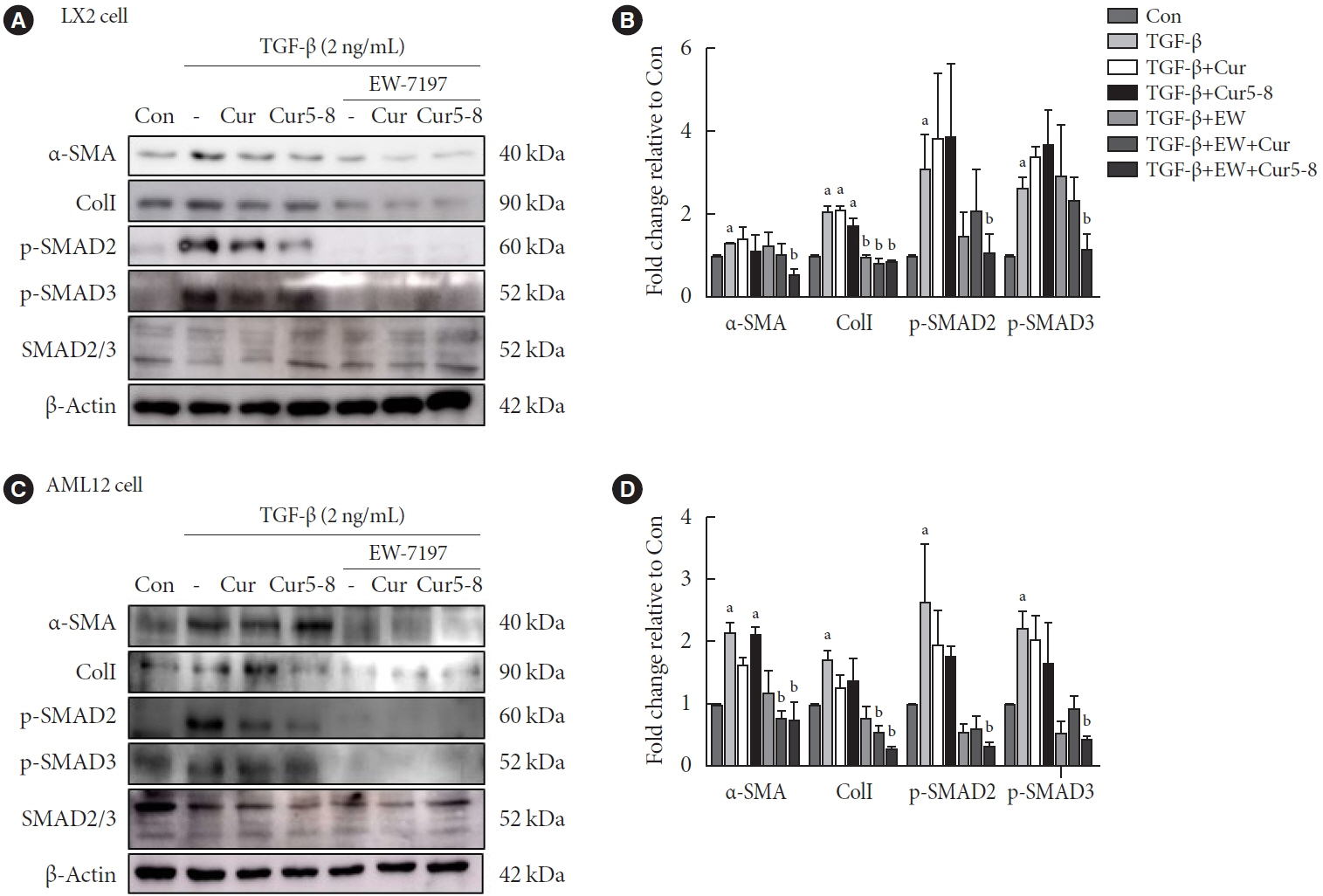
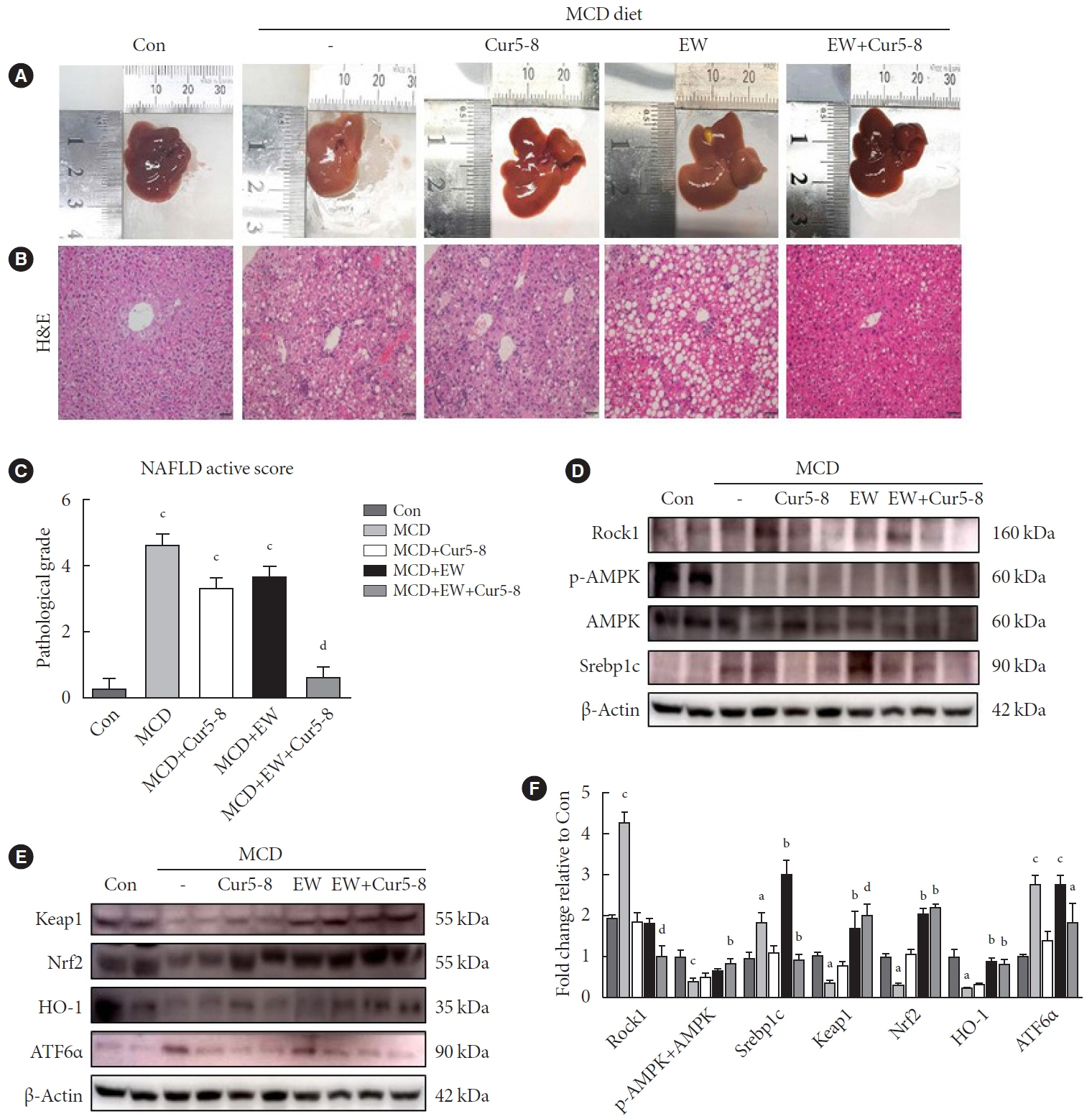
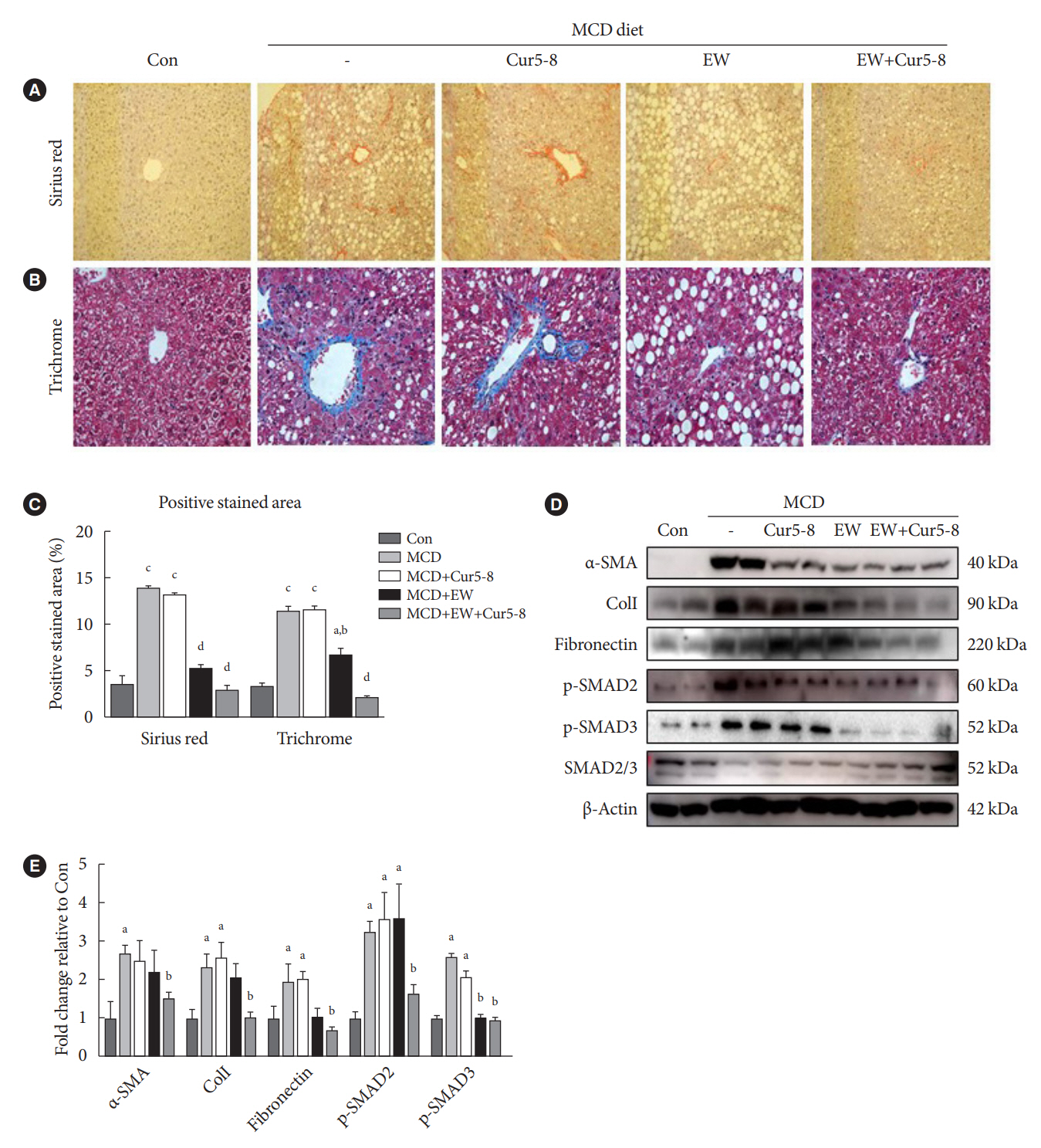
TGF-β-induced cell morphological changes were improved by EW-7197, and lipid accumulation was restored on the administration of EW-7197 in combination with Cur5-8. In a nonalcoholic steatohepatitis (NASH)-induced mouse model, 6 weeks of EW-7197 and Cur5-8 co-administration alleviated liver fibrosis and improved the nonalcoholic fatty liver disease (NAFLD) activity score.
Conclusion
Co-administering Cur5-8 and EW-7197 to NASH-induced mice and fibrotic hepatocytes reduced liver fibrosis and steatohepatitis while maintaining the advantages of both drugs. This is the first study to show the effect of the drug combination against NASH and NAFLD. Similar effects in other animal models will confirm its potential as a new therapeutic agent.
Keyword
Figure
Reference
-
1. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019; 71:793–801.
Article2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64:73–84.
Article3. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018; 67:328–57.
Article4. Dufour JF, Caussy C, Loomba R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut. 2020; 69:1877–84.
Article5. Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007; 117:539–48.
Article6. Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008; 134:1655–69.
Article7. Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004; 18:816–27.8. Liu RM, Gaston Pravia KA. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic Biol Med. 2010; 48:1–15.9. Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000; 275:2247–50.
Article10. Ooshima A, Park J, Kim SJ. Phosphorylation status at Smad3 linker region modulates transforming growth factor-βinduced epithelial-mesenchymal transition and cancer progression. Cancer Sci. 2019; 110:481–8.
Article11. Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000; 19:1745–54.12. Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003; 113:685–700.
Article13. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003; 425:577–84.
Article14. Panahi Y, Hosseini MS, Khalili N, Naimi E, Simental-Mendia LE, Majeed M, et al. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: a posthoc analysis of a randomized controlled trial. Biomed Pharmacother. 2016; 82:578–82.
Article15. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, Buntragulpoontawee M, Lukkanapichonchut P, Chootip C, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014; 9:451–8.
Article16. Jager R, Lowery RP, Calvanese AV, Joy JM, Purpura M, Wilson JM. Comparative absorption of curcumin formulations. Nutr J. 2014; 13:11.
Article17. Lee ES, Kwon MH, Kim HM, Woo HB, Ahn CM, Chung CH. Curcumin analog CUR5-8 ameliorates nonalcoholic fatty liver disease in mice with high-fat diet-induced obesity. Metabolism. 2020; 103:154015.
Article18. Jin CH, Krishnaiah M, Sreenu D, Subrahmanyam VB, Rao KS, Lee HJ, et al. Discovery of N-((4-([1,2,4]triazolo[1,5-a]pyridin6-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2-yl)methyl)- 2-fluoroaniline (EW-7197): a highly potent, selective, and orally bioavailable inhibitor of TGF-β type I receptor kinase as cancer immunotherapeutic/antifibrotic agent. J Med Chem. 2014; 57:4213–38.19. Park SA, Kim MJ, Park SY, Kim JS, Lee SJ, Woo HA, et al. EW-7197 inhibits hepatic, renal, and pulmonary fibrosis by blocking TGF-β/Smad and ROS signaling. Cell Mol Life Sci. 2015; 72:2023–39.
Article20. Kim MJ, Park SA, Kim CH, Park SY, Kim JS, Kim DK, et al. TGF-β type I receptor kinase inhibitor EW-7197 suppresses cholestatic liver fibrosis by inhibiting HIF1α-induced epithelial mesenchymal transition. Cell Physiol Biochem. 2016; 38:571–88.
Article21. Ha KB, Sangartit W, Jeong AR, Lee ES, Kim HM, Shim S, et al. EW-7197 attenuates the progression of diabetic nephropathy in db/db mice through suppression of fibrogenesis and inflammation. Endocrinol Metab (Seoul). 2022; 37:96–111.
Article22. Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, et al. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD1. J Lipid Res. 2006; 47:2280–90.
Article23. Josan S, Billingsley K, Orduna J, Park JM, Luong R, Yu L, et al. Assessing inflammatory liver injury in an acute CCl4 model using dynamic 3D metabolic imaging of hyperpolarized [1-(13)C]pyruvate. NMR Biomed. 2015; 28:1671–7.24. Lepreux S, Desmouliere A. Human liver myofibroblasts during development and diseases with a focus on portal (myo)fibroblasts. Front Physiol. 2015; 6:173.
Article25. Issa R, Zhou X, Constandinou CM, Fallowfield J, MillwardSadler H, Gaca MD, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004; 126:1795–808.
Article26. Popov Y, Sverdlov DY, Sharma AK, Bhaskar KR, Li S, Freitag TL, et al. Tissue transglutaminase does not affect fibrotic matrix stability or regression of liver fibrosis in mice. Gastroenterology. 2011; 140:1642–52.
Article27. Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009; 50:1294–306.
Article28. Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010; 7:425–36.
Article29. van Dijk F, Olinga P, Poelstra K, Beljaars L. Targeted therapies in liver fibrosis: combining the best parts of platelet-derived growth factor BB and interferon gamma. Front Med (Lausanne). 2015; 2:72.
Article30. Viollet B, Lantier L, Devin-Leclerc J, Hebrard S, Amouyal C, Mounier R, et al. Targeting the AMPK pathway for the treatment of type 2 diabetes. Front Biosci (Landmark Ed). 2009; 14:3380–400.
Article31. Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive elementbinding protein by AMP-activated protein kinase. J Biol Chem. 2002; 277:3829–35.32. Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011; 13:376–88.
Article33. Hannon GJ, Beach D. p15INK4B is a potential effector of TGFbeta-induced cell cycle arrest. Nature. 1994; 371:257–61.
Article34. Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci U S A. 1995; 92:5545–9.
Article35. Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006; 87:1–16.
Article36. Chowdhry S, Nazmy MH, Meakin PJ, Dinkova-Kostova AT, Walsh SV, Tsujita T, et al. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med. 2010; 48:357–71.
Article37. Larter CZ, Yeh MM, Haigh WG, Williams J, Brown S, Bell-Anderson KS, et al. Hepatic free fatty acids accumulate in experimental steatohepatitis: role of adaptive pathways. J Hepatol. 2008; 48:638–47.
Article38. Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000; 105:1067–75.
Article39. Juluri R, Vuppalanchi R, Olson J, Unalp A, Van Natta ML, Cummings OW, et al. Generalizability of the nonalcoholic steatohepatitis Clinical Research Network histologic scoring system for nonalcoholic fatty liver disease. J Clin Gastroenterol. 2011; 45:55–8.
Article40. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41:1313–21.
Article41. Asselah T, Marcellin P, Bedossa P. Improving performance of liver biopsy in fibrosis assessment. J Hepatol. 2014; 61:193–5.
Article42. Huang H, Lee SH, Sousa-Lima I, Kim SS, Hwang WM, Dagon Y, et al. Rho-kinase/AMPK axis regulates hepatic lipogenesis during overnutrition. J Clin Invest. 2018; 128:5335–50.
Article43. Gupta J, Gaikwad AB, Tikoo K. Hepatic expression profiling shows involvement of PKC epsilon, DGK eta, Tnfaip, and Rho kinase in type 2 diabetic nephropathy rats. J Cell Biochem. 2010; 111:944–54.
Article44. Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007; 68:72–82.
Article45. Wu W, Tang S, Shi J, Yin W, Cao S, Bu R, et al. Metformin attenuates palmitic acid-induced insulin resistance in L6 cells through the AMP-activated protein kinase/sterol regulatory element-binding protein-1c pathway. Int J Mol Med. 2015; 35:1734–40.
Article46. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–29.
Article47. Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006; 46:113–40.
Article48. Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999; 274:26071–8.
Article49. Yen IC, Tu QW, Chang TC, Lin PH, Li YF, Lee SY. 4-Acetylantroquinonol B ameliorates nonalcoholic steatohepatitis by suppression of ER stress and NLRP3 inflammasome activation. Biomed Pharmacother. 2021; 138:111504.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An experimental study on the stimulatory effects of epidermal growth factor and transforming growth factor-alpha on the growth of squamous cancer cell lines
- Role of Transforming Growth Factor Alpha in Hepatocarcinogenesis
- Expressions of transforming growth factor beta in patients with rheumatioid arthritis and osteoarthritis
- Curcumin Induced Decreased Expression of Type I Collagen in Human Skin Fibroblast Through Down-regulation of Smad2/3 Expressions
- IL-10 Inihibits VEGF Production in the Synovial Fibroblasts of RA Patients via Down-regulation of the ERK and AP-1 Pathways