Clin Endosc.
2023 Jul;56(4):460-469. 10.5946/ce.2022.167.
Exploring quality indicators for the detection of Helicobacter pylori-naïve gastric cancer: a cross-sectional nationwide survey
- Affiliations
-
- 1Department of Gastroenterology, International University of Health and Welfare Ichikawa Hospital, Chiba
- 2Endoscopy Center, Koganei Tsurukame Clinic, Tokyo, Japan
- 3Department of Gastroenterology, Cancer Institute Hospital, Tokyo, Japan
- 4Department of Gastroenterology, Juntendo University School of Medicine, Tokyo, Japan
- 5Department of Gastrointestinal Endoscopy, NTT Medical Center Tokyo, Tokyo, Japan
- KMID: 2544569
- DOI: http://doi.org/10.5946/ce.2022.167
Abstract
- Background/Aims
Diagnosis of Helicobacter pylori-naïve gastric cancer (HPNGC) is becoming increasingly important. This study aimed to explore the quality indicators for HPNGC detection.
Methods
We conducted a cross-sectional, nationwide, web-based survey of gastrointestinal endoscopists in Japan. In addition to questions about the number of HPNGC cases detected in a year and basic information, the questionnaire also consisted of 28 questions: (1) 18 about HPNGC awareness, (2) six about diagnostic proactiveness, and (3) four about interest in HPNGC.
Results
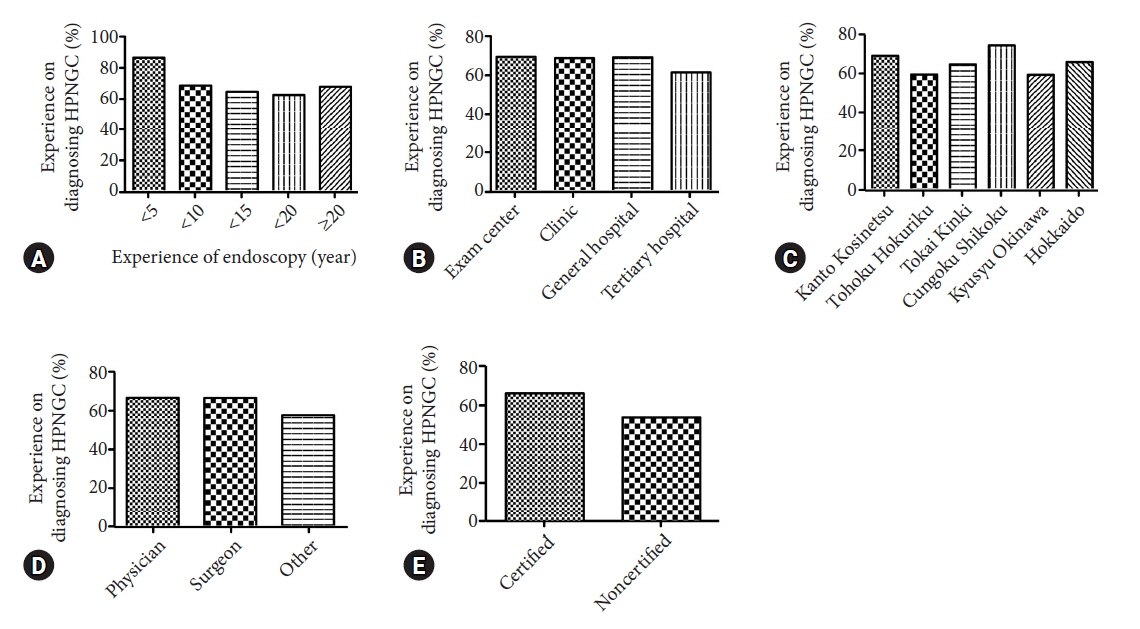
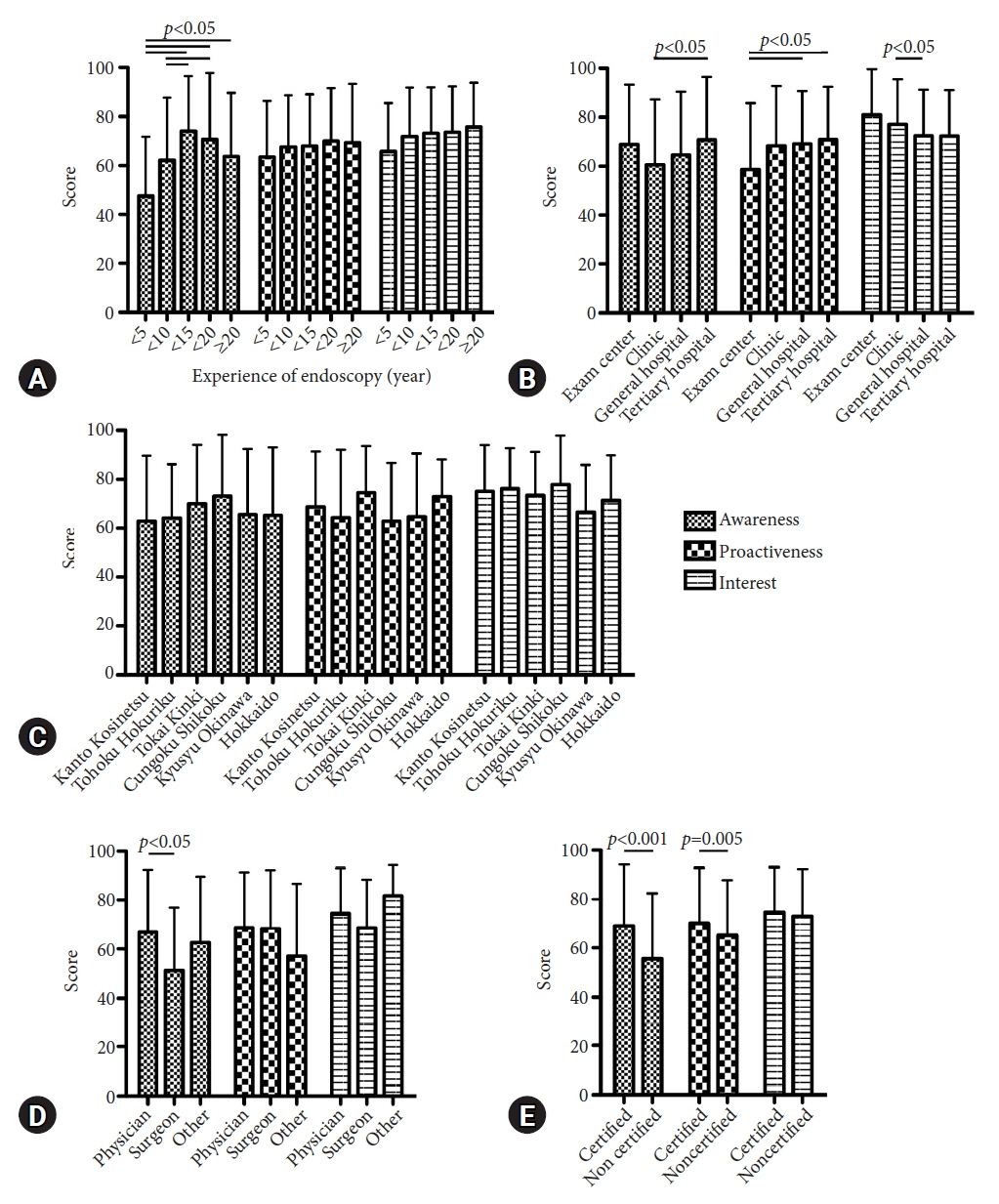
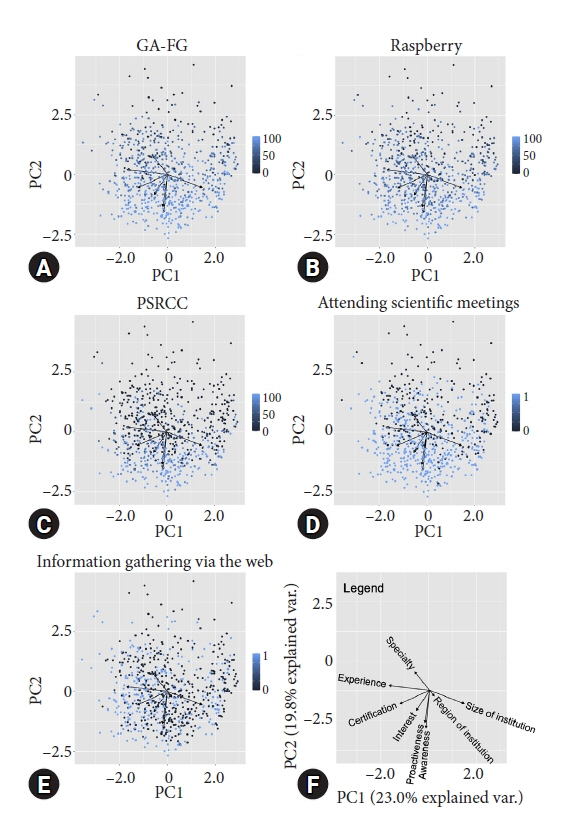
Valid responses were obtained from 712 endoscopists. The Japan Gastroenterological Endoscopy Society-certified endoscopists had a significantly higher HPNGC detection rate than the nonspecialists (0.42% vs. 0.32%, respectively; p=0.008). The results of the multiple regression analysis showed that Japan Gastroenterological Endoscopy Society certification and high awareness and interest scores were independent predictors of the HPNGC detection rate (p=0.012, p<0.001, p=0.024, respectively). Principal component analysis showed that the endoscopists who attended conferences for collecting information on HPNGC had a higher level of awareness.
Conclusions
To improve the detection of HPNGC, it is necessary to increase the awareness of the disease. It is hoped that relevant societies will play an important role in endoscopists’ education.
Keyword
Figure
Reference
-
1. Nomura A, Stemmermann GN, Chyou PH, et al. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991; 325:1132–1136.2. Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001; 49:347–353.3. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008; 372:392–397.4. Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018; 378:1085–1095.5. Inoue M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer. 2017; 20(Suppl 1):3–7.6. Wang C, Nishiyama T, Kikuchi S, et al. Changing trends in the prevalence of H. pylori infection in Japan (1908-2003): a systematic review and meta-regression analysis of 170,752 individuals. Sci Rep. 2017; 7:15491.7. Watanabe M, Ito H, Hosono S, et al. Declining trends in prevalence of Helicobacter pylori infection by birth-year in a Japanese population. Cancer Sci. 2015; 106:1738–1743.8. Matsuo T, Ito M, Takata S, et al. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter. 2011; 16:415–419.9. Kiso M, Yoshihara M, Ito M, et al. Characteristics of gastric cancer in negative test of serum anti-Helicobacter pylori antibody and pepsinogen test: a multicenter study. Gastric Cancer. 2017; 20:764–771.10. Nikaido M, Kakiuchi N, Miyamoto S, et al. Indolent feature of Helicobacter pylori-uninfected intramucosal signet ring cell carcinomas with CDH1 mutations. Gastric Cancer. 2021; 24:1102–1114.11. Kiso M, Urabe Y, Ito M, et al. Clinical and genomic characteristics of mucosal signet-ring cell carcinoma in Helicobacter pylori-uninfected stomach. BMC Gastroenterol. 2020; 20:243.12. Ueyama H, Yao T, Nakashima Y, et al. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010; 34:609–619.13. Ueyama H, Matsumoto K, Nagahara A, et al. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014; 46:153–157.14. Ueyama H, Yao T, Akazawa Y, et al. Gastric epithelial neoplasm of fundic-gland mucosa lineage: proposal for a new classification in association with gastric adenocarcinoma of fundic-gland type. J Gastroenterol. 2021; 56:814–828.15. Ishibashi F, Fukushima K, Ito T, et al. Influence of Helicobacter pylori infection on endoscopic findings of gastric adenocarcinoma of the fundic gland type. J Gastric Cancer. 2019; 19:225–233.16. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.17. Shibagaki K, Fukuyama C, Mikami H, et al. Gastric foveolar-type adenomas endoscopically showing a raspberry-like appearance in the Helicobacter pylori-uninfected stomach. Endosc Int Open. 2019; 7:E784–E791.18. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965; 64:31–49.19. Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumors of the digestive system. 4th ed. Lyon: JARC;2010.20. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020; 76:182–188.21. Sato C, Hirasawa K, Tateishi Y, et al. Clinicopathological features of early gastric cancers arising in Helicobacter pylori uninfected patients. World J Gastroenterol. 2020; 26:2618–2631.22. Ishibashi F, Kobayashi K, Fukushima K, et al. Quality indicators for the detection of Helicobacter pylori-negative early gastric cancer: a retrospective observational study. Clin Endosc. 2020; 53:698–704.23. Ishibashi F, Kobayashi K, Kawakami T, et al. Quality management system for screening esophagogastroduodenoscopy improves detection of Helicobacter pylori-negative interval gastric cancer. Endosc Int Open. 2021; 9:E1900–E1908.24. Lee SY, Yoshida N, Dohi O, et al. Differences in prevalence of lymphovascular invasion among early gastric cancers between Korea and Japan. Gut Liver. 2017; 11:383–391.25. R Core Team. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing;2022 [cited 2022 Mar 3]. Available from: https://www.R-project.org/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Helicobacter pylori-negative Gastric Cancer
- Approach to Patients after Successful Eradication of Helicobacter pylori
- Quality Indicators for the Detection of Helicobacter pylori-Negative Early Gastric Cancer: A Retrospective Observational Study
- Exploring the Link between Helicobacter pylori Eradication and Metachronous Gastric Cancer Development
- Guidelines for Helicobacter pylori Eradication