Obstet Gynecol Sci.
2023 Jul;66(4):307-315. 10.5468/ogs.23103.
Triglyceride and glucose index for identifying abnormal insulin sensitivity in women with polycystic ovary syndrome
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- KMID: 2544386
- DOI: http://doi.org/10.5468/ogs.23103
Abstract
Objective
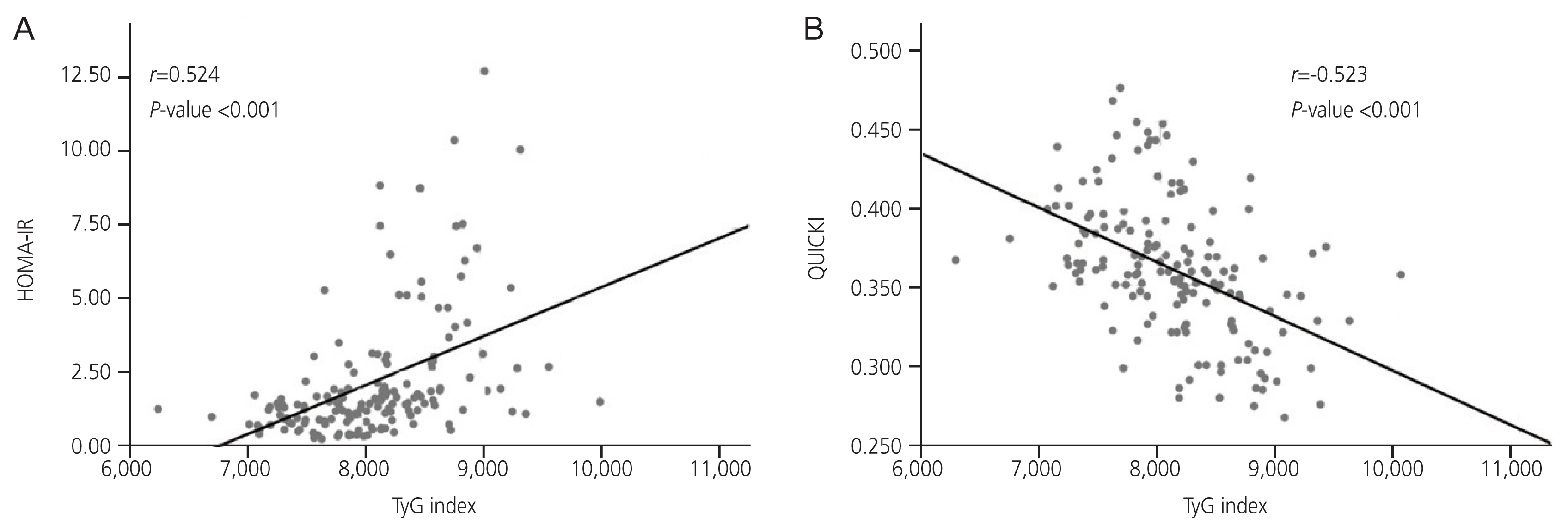
We aimed to evaluate whether triglyceride and glucose (TyG) indices are useful in identifying insulin sensitivity/resistance in women with polycystic ovary syndrome (PCOS).
Methods
One hundred and seventy-two Korean women aged 18-35 years who were diagnosed with PCOS were included in this study. Fasting-state insulin sensitivity assessment indices (ISAIs) derived from a combination of fasting insulin and glucose levels were calculated for all study participants, and abnormal insulin sensitivity was defined as any of the evaluated ISAIs being out of the established normal range. Correlation analysis was conducted to assess the relationship between the TyG index and other clinical and biochemical parameters. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff value of the TyG index for identifying abnormal insulin sensitivity, and unpaired t-tests were used to compare biochemical parameters between individuals with a TyG index below the cutoff and individuals with a TyG index above the cutoff value.
Results
All clinical parameters, except age and other insulin resistance-related biochemical parameters, were significantly related to the TyG index. The ROC curve analysis revealed an optimal TyG cutoff value of 8.126 (sensitivity, 0.807; specificity, 0.683) for identifying abnormal insulin sensitivity. In the comparative analysis, all ISAIs and parameters derived from the lipid profiles differed significantly between the TyG groups.
Conclusion
The TyG index is a feasible surrogate marker for predicting insulin sensitivity/resistance in women with PCOS.
Keyword
Figure
Reference
-
References
1. Peterson KR, Link M, Peterson CM. Endocrine disorder. Berek JS, editor. Berek & Novak’s gynecology. 16th ed.Philadelphia: Lippincott Williams & Wilkins;2019. p. 889–941.2. McCartney CR, Marshall JC. Clinical practice: polycystic ovary syndrome. N Engl J Med. 2016; 375:54–64.3. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005; 352:1223–36.
Article4. Carmina E, Lobo RA. Use of fasting blood to assess the prevalence of insulin resistance in women with polycystic ovary syndrome. Fertil Steril. 2004; 82:661–5.
Article5. Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab. 2003; 14:365–70.
Article6. Chun S. Predictive capability of fasting-state glucose and insulin measurements for abnormal glucose tolerance in women with polycystic ovary syndrome. Clin Exp Reprod Med. 2021; 48:156–62.
Article7. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997; 18:774–800.
Article8. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008; 294:E15–26.
Article9. Kim JJ, Hwang KR, Oh SH, Chae SJ, Yoon SH, Choi YM. Prevalence of insulin resistance in Korean women with polycystic ovary syndrome according to various homeostasis model assessment for insulin resistance cutoff values. Fertil Steril. 2019; 112:959–66.
Article10. Son DH, Ha HS, Park HM, Kim HY, Lee YJ. New markers in metabolic syndrome. Adv Clin Chem. 2022; 110:37–71.
Article11. Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013; 98:4565–92.
Article12. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2019; 34:388.
Article13. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006; 23:469–80.
Article14. Krawczyk M, Rumińska M, Witkowska-Sędek E, Majcher A, Pyrżak B. Usefulness of the triglycerides to high-density lipoprotein cholesterol ratio (TG/HDL-C) in prediction of metabolic syndrome in Polish obese children and adolescents. Acta Biochim Pol. 2018; 65:605–11.
Article15. Blum MR, Popat RA, Nagy A, Cataldo NA, McLaughlin TL. Using metabolic markers to identify insulin resistance in premenopausal women with and without polycystic ovary syndrome. J Endocrinol Invest. 2021; 44:2123–30.
Article16. Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the vascular-metabolic CUN cohort. Prev Med. 2016; 86:99–105.
Article17. Moon S, Park JS, Ahn Y. The cut-off values of triglycerides and glucose index for metabolic syndrome in American and Korean adolescents. J Korean Med Sci. 2017; 32:427–33.
Article18. Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review. Int J Endocrinol. 2020; 2020:4678526.
Article19. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008; 6:299–304.
Article20. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010; 95:3347–51.
Article21. Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014; 13:146.
Article22. Song do K, Lee H, Sung YA, Oh JY. Triglycerides to high-density lipoprotein cholesterol ratio can predict impaired glucose tolerance in young women with polycystic ovary syndrome. Yonsei Med J. 2016; 57:1404–11.
Article23. Ghaffarzad A, Amani R, Mehrzad Sadaghiani M, Darabi M, Cheraghian B. Correlation of serum lipoprotein ratios with insulin resistance in infertile women with polycystic ovarian syndrome: a case control study. Int J Fertil Steril. 2016; 10:29–35.24. Xiang SK, Hua F, Tang Y, Jiang XH, Zhuang Q, Qian FJ. Relationship between serum lipoprotein ratios and insulin resistance in polycystic ovary syndrome. Int J Endocrinol. 2012; 2012:173281.
Article25. Ulutaş F, Cander S, Özen ÖZ. The association between triglycerides/high-density lipoprotein cholesterol ratio, insulin resistance, and serum androgen levels in patients with polycystic ovary syndrome. Eur Res J. 2022; 8:275–81.
Article26. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004; 19:41–7.27. Chun S. 1-h postprandial glucose level is related to the serum anti-müllerian hormone level in women with polycystic ovary syndrome. Gynecol Endocrinol. 2015; 31:815–8.
Article28. Park CH, Chun S. Association between serum gonadotropin level and insulin resistance-related parameters in Korean women with polycystic ovary syndrome. Obstet Gynecol Sci. 2016; 59:498–505.
Article29. Park CH, Chun S. Influence of combined oral contraceptives on polycystic ovarian morphology-related parameters in Korean women with polycystic ovary syndrome. Obstet Gynecol Sci. 2020; 63:80–6.
Article30. Kim N, Chun S. Association between the serum estrone-to-estradiol ratio and parameters related to glucose metabolism and insulin resistance in women with polycystic ovary syndrome. Clin Exp Reprod Med. 2021; 48:374–9.
Article31. Chun S, Lee S. Optimal cutoff value of 1-hour postload glucose to identify insulin resistance in women with polycystic ovary syndrome. Clin Exp Obstet Gynecol. 2022; 49:219.
Article32. Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003; 9:505–14.
Article33. Negishi H, Nakao K, Kimura M, Takenaka H, Horikawa M. Insulin resistance in nonobese Japanese women with polycystic ovary syndrome is associated with poorer glucose tolerance, delayed insulin secretion, and enhanced insulin response. Reprod Med Biol. 2015; 14:123–9.
Article34. Chen X, Yang D, Li L, Feng S, Wang L. Abnormal glucose tolerance in Chinese women with polycystic ovary syndrome. Hum Reprod. 2006; 21:2027–32.
Article35. Consensus development conference on insulin resistance. 5–6 November 1997. American Diabetes Association. Diabetes Care. 1998; 21:310–4.36. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–97.37. Kheirollahi A, Teimouri M, Karimi M, Vatannejad A, Moradi N, Borumandnia N, et al. Evaluation of lipid ratios and triglyceride-glucose index as risk markers of insulin resistance in Iranian polycystic ovary syndrome women. Lipids Health Dis. 2020; 19:235.
Article38. Li R, Li Q, Cui M, Yin Z, Li L, Zhong T, et al. Clinical surrogate markers for predicting metabolic syndrome in middle-aged and elderly Chinese. J Diabetes Investig. 2018; 9:411–8.
Article39. Zheng Y, Yin G, Chen F, Lin L, Chen Y. Evaluation of triglyceride glucose index and homeostasis model of insulin resistance in patients with polycystic ovary syndrome. Int J Womens Health. 2022; 14:1821–9.
Article40. Bilginer MC, Tufekci D, Gunay YE, Usta O, Coskun H, Ucuncu O, et al. Evaluation of insulin resistance measurement methods in patients with polycystic ovary sdyndrome. Turk J Diab Obes. 2022; 1:24–31.41. Alizargar J, Hsieh NC, Wu SV. The correct formula to calculate triglyceride-glucose index (TyG). J Pediatr Endocrinol Metab. 2020; 33:945–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Oral Glucose Insulin Sensitivity Index in Women with Polycystic Ovary Syndrome
- Predictive capability of fasting-state glucose and insulin measurements for abnormal glucose tolerance in women with polycystic ovary syndrome
- Polycystic Ovary Syndrome in Korean Women: Clinical Characteristics and Diagnostic Criteria
- Predictors of abnormal glucose tolerance among women with polycystic ovary syndrome
- Association of Serum Adiponectin Levels with Insulin Resistance in Women with Polycystic Ovary Syndrome