Acute Crit Care.
2023 May;38(2):172-181. 10.4266/acc.2022.01494.
Relationship between positive end-expiratory pressure levels, central venous pressure, systemic inflammation and acute renal failure in critically ill ventilated COVID-19 patients: a monocenter retrospective study in France
- Affiliations
-
- 1Department of Anesthesiology and Critical Care Medicine, AP-HP, Hôpital Lariboisière, Paris, France
- 2Department of Anesthesiology and Pain Medicine, Hôpital Maisonneuve-Rosemont, CIUSSS de l’Est de l’Ile de Montréal, Montréal, Canada
- 3Université Paris-Cité, INSERM, U942 MASCOT, Paris, France
- KMID: 2543636
- DOI: http://doi.org/10.4266/acc.2022.01494
Abstract
- Background
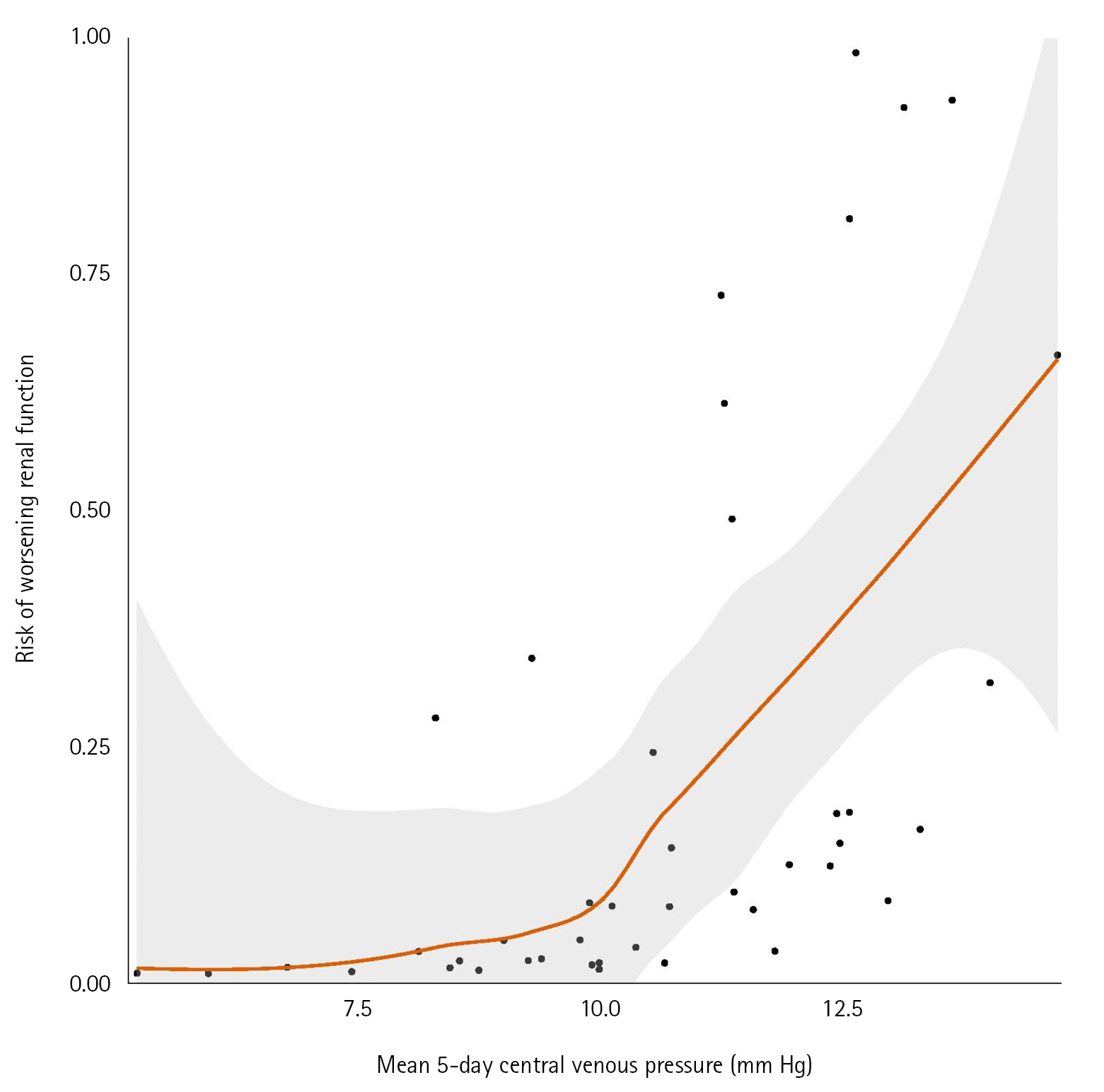
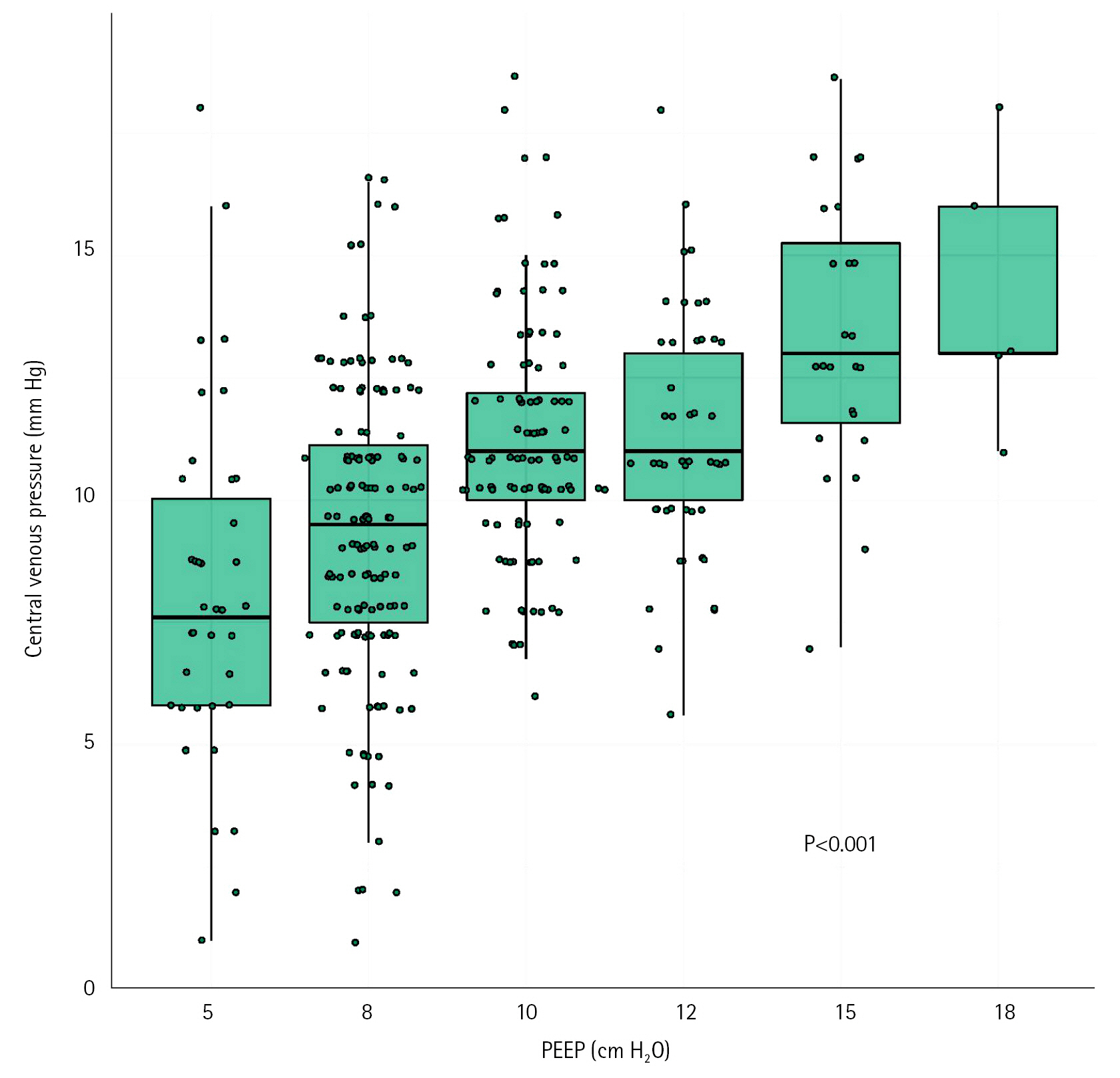
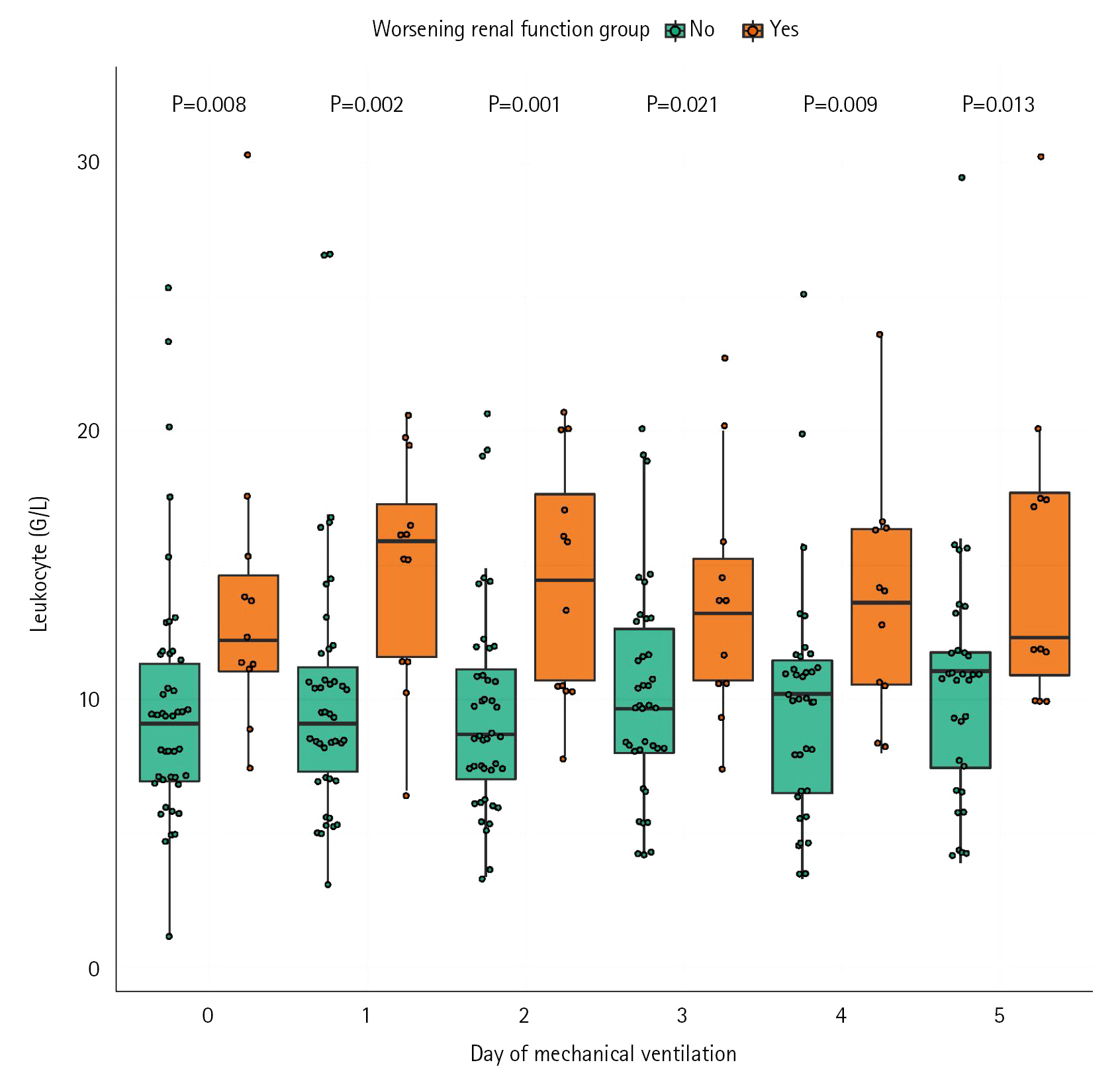
The role of positive pressure ventilation, central venous pressure (CVP) and inflammation on the occurrence of acute kidney injury (AKI) have been poorly described in mechanically ventilated patient secondary to coronavirus disease 2019 (COVID-19). Methods: This was a monocenter retrospective cohort study of consecutive ventilated COVID-19 patients admitted in a French surgical intensive care unit between March 2020 and July 2020. Worsening renal function (WRF) was defined as development of a new AKI or a persistent AKI during the 5 days after mechanical ventilation initiation. We studied the association between WRF and ventilatory parameters including positive end-expiratory pressure (PEEP), CVP, and leukocytes count. Results: Fifty-seven patients were included, 12 (21%) presented WRF. Daily PEEP, 5 days mean PEEP and daily CVP values were not associated with occurrence of WRF. 5 days mean CVP was higher in the WRF group compared to patients without WRF (median [IQR], 12 mm Hg [11-13] vs. 10 mm Hg [9–12]; P=0.03). Multivariate models with adjustment on leukocytes and Simplified Acute Physiology Score (SAPS) II confirmed the association between CVP value and risk of WRF (odd ratio, 1.97; 95% confidence interval, 1.12–4.33). Leukocytes count was also associated with occurrence of WRF in the WRF group (14 G/L [11–18]) and the no-WRF group (9 G/L [8–11]) (P=0.002). Conclusions: In mechanically ventilated COVID-19 patients, PEEP levels did not appear to influence occurrence of WRF. High CVP levels and leukocytes count are associated with risk of WRF.
Keyword
Figure
Reference
-
1. Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021; 32:151–60.
Article2. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708–20.
Article3. Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020; 98:209–18.4. Xu Z, Tang Y, Huang Q, Fu S, Li X, Lin B, et al. Systematic review and subgroup analysis of the incidence of acute kidney injury (AKI) in patients with COVID-19. BMC Nephrol. 2021; 22:52.
Article5. Chang R, Elhusseiny KM, Yeh YC, Sun WZ. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes: a systematic review and meta-analysis. PLoS One. 2021; 16:e0246318.6. Costa RL, Sória TC, Salles EF, Gerecht AV, Corvisier MF, Menezes MA, et al. Acute kidney injury in patients with Covid-19 in a Brazilian ICU: incidence, predictors and in-hospital mortality. J Bras Nefrol. 2021; 43:349–58.
Article7. Yang X, Tian S, Guo H. Acute kidney injury and renal replacement therapy in COVID-19 patients: a systematic review and meta-analysis. Int Immunopharmacol. 2021; 90:107159.
Article8. Hultström M, Lipcsey M, Wallin E, Larsson IM, Larsson A, Frithiof R. Severe acute kidney injury associated with progression of chronic kidney disease after critical COVID-19. Crit Care. 2021; 25:37.
Article9. Hamilton P, Hanumapura P, Castelino L, Henney R, Parker K, Kumar M, et al. Characteristics and outcomes of hospitalised patients with acute kidney injury and COVID-19. PloS One. 2020; 15:e0241544.
Article10. Doher MP, Torres de Carvalho FR, Scherer PF, Matsui TN, Ammirati AL, Caldin da Silva B, et al. Acute kidney injury and renal replacement therapy in critically ill COVID-19 patients: risk factors and outcomes: a single-center experience in Brazil. Blood Purif. 2021; 50:520–30.
Article11. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181:271–80.
Article12. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020; 14:185–92.
Article13. Migliaccio MG, Di Mauro M, Ricciolino R, Spiniello G, Carfora V, Verde N, et al. Renal involvement in COVID-19: a review of the literature. Infect Drug Resist. 2021; 14:895–903.
Article14. Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020; 31:1380–3.
Article15. Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021; 17:751–64.
Article16. Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021; 93:250–6.
Article17. Mullins RJ, Dawe EJ, Lucas CE, Ledgerwood AM, Banks SM. Mechanisms of impaired renal function with PEEP. J Surg Res. 1984; 37:189–96.
Article18. Priebe HJ, Heimann JC, Hedley-Whyte J. Mechanisms of renal dysfunction during positive end-expiratory pressure ventilation. J Appl Physiol Respir Environ Exerc Physiol. 1981; 50:643–9.
Article19. Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013; 17:R278.
Article20. Chen KP, Cavender S, Lee J, Feng M, Mark RG, Celi LA, et al. Peripheral edema, central venous pressure, and risk of AKI in critical illness. Clin J Am Soc Nephrol. 2016; 11:602–8.
Article21. Geri G, Ferrer L, Tran N, Celi LA, Jamme M, Lee J, et al. Cardio-pulmonary-renal interactions in ICU patients: role of mechanical ventilation, venous congestion and perfusion deficit on worsening of renal function: insights from the MIMIC-III database. J Crit Care. 2021; 64:100–7.
Article22. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993; 270:2957–63.
Article23. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996; 22:707–10.
Article24. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012; 307:2526–33.25. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012; 120:c179–84.
Article26. Kork F, Balzer F, Krannich A, Bernardi MH, Eltzschig HK, Jankowski J, et al. Back-calculating baseline creatinine overestimates prevalence of acute kidney injury with poor sensitivity. Acta Physiol (Oxf). 2017; 219:613–24.
Article27. Ottolina D, Zazzeron L, Trevisi L, Agarossi A, Colombo R, Fossali T, et al. Acute kidney injury (AKI) in patients with Covid-19 infection is associated with ventilatory management with elevated positive end-expiratory pressure (PEEP). J Nephrol. 2022; 35:99–111.
Article28. Beurton A, Haudebourg L, Simon-Tillaux N, Demoule A, Dres M. Limiting positive end-expiratory pressure to protect renal function in SARS-CoV-2 critically ill patients. J Crit Care. 2020; 59:191–3.
Article29. Chaibi K, Dao M, Pham T, Gumucio-Sanguino VD, Di Paolo FA, Pavot A, et al. Severe acute kidney injury in patients with COVID-19 and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020; 202:1299–301.
Article30. van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care. 2013; 17:R98.
Article31. Barthélémy R, Beaucoté V, Bordier R, Collet M, Le Gall A, Hong A, et al. Haemodynamic impact of positive end-expiratory pressure in SARS-CoV-2 acute respiratory distress syndrome: oxygenation versus oxygen delivery. Br J Anaesth. 2021; 126:e70–2.
Article32. Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008; 108:735–48.33. Vieillard-Baron A, Matthay M, Teboul JL, Bein T, Schultz M, Magder S, et al. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med. 2016; 42:739–49.
Article34. Jardin F, Vieillard-Baron A. Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. Intensive Care Med. 2003; 29:1426–34.
Article35. Hardenberg JB, Stockmann H, Aigner A, Gotthardt I, Enghard P, Hinze C, et al. Critical illness and systemic inflammation are key risk factors of severe acute kidney injury in patients with COVID-19. Kidney Int Rep. 2021; 6:905–15.
Article36. Lumlertgul N, Pirondini L, Cooney E, Kok W, Gregson J, Camporota L, et al. Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: a cohort study. Ann Intensive Care. 2021; 11:123.
Article37. Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014; 40:1795–815.
Article38. Susen S, Tacquard CA, Godon A, Mansour A, Garrigue D, Nguyen P, et al. Prevention of thrombotic risk in hospitalized patients with COVID-19 and hemostasis monitoring. Crit Care. 2020; 24:364.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Positive End-Expiratory Pressure on Intraocular Pressure in the Critically Ill and Mechanically Ventilated Patients
- Changes of Central Venous Pressure following the Induction of General Anesthesia in Patients with End-Stage Renal Disease
- The Effect of Positive end Expiratory pressure on the Pulmonary Capillary Pressure in Acute Lung Injury Patients
- The changes of central venous pressure by body posture and positive end-expiratory pressure
- Monitoring of Arterial and Central Venous Pressure in Critically Ill Patients