Acute Crit Care.
2023 May;38(2):160-171. 10.4266/acc.2022.01424.
Comparison of safety and efficacy between therapeutic or intermediate versus prophylactic anticoagulation for thrombosis in COVID-19 patients: a systematic review and meta-analysis
- Affiliations
-
- 1Division of Healthcare Technology Assessment Research, National Evidence-based Healthcare Collaborating Agency, Seoul, Korea
- 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Myongji Hospital, Hanyang University, Korea
- 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, National Medical Center, Seoul, Korea
- KMID: 2543635
- DOI: http://doi.org/10.4266/acc.2022.01424
Abstract
- Background
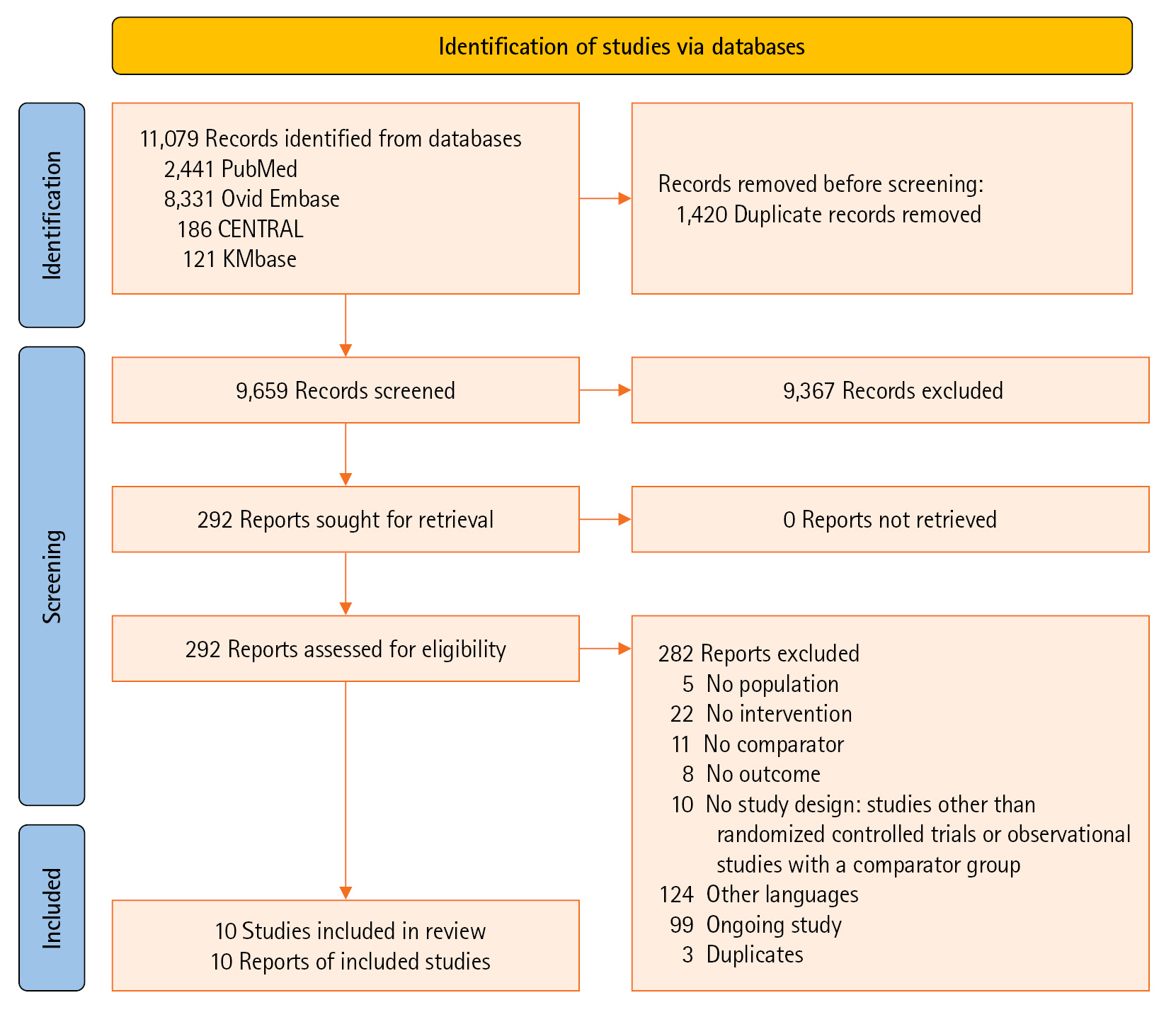
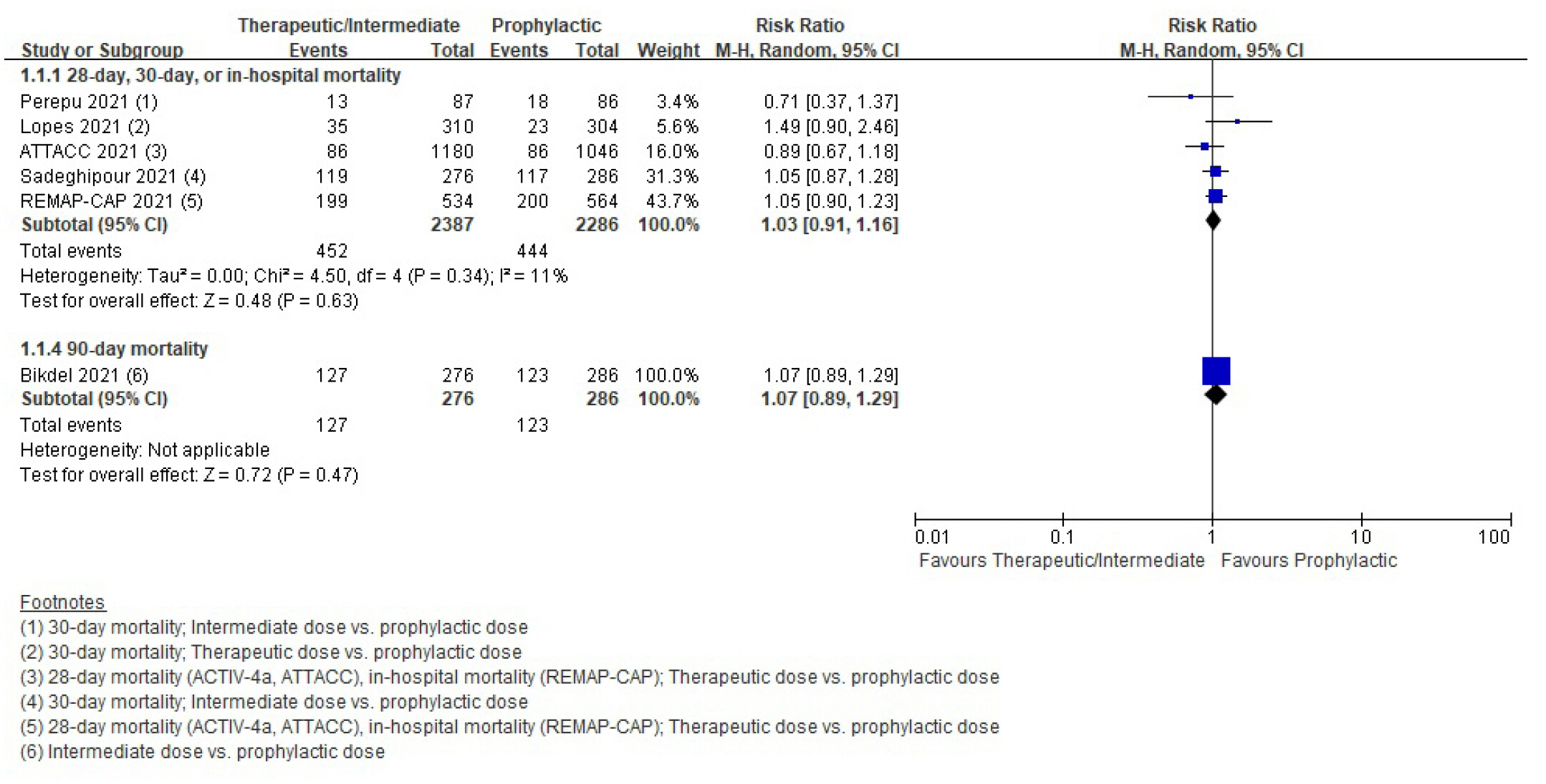
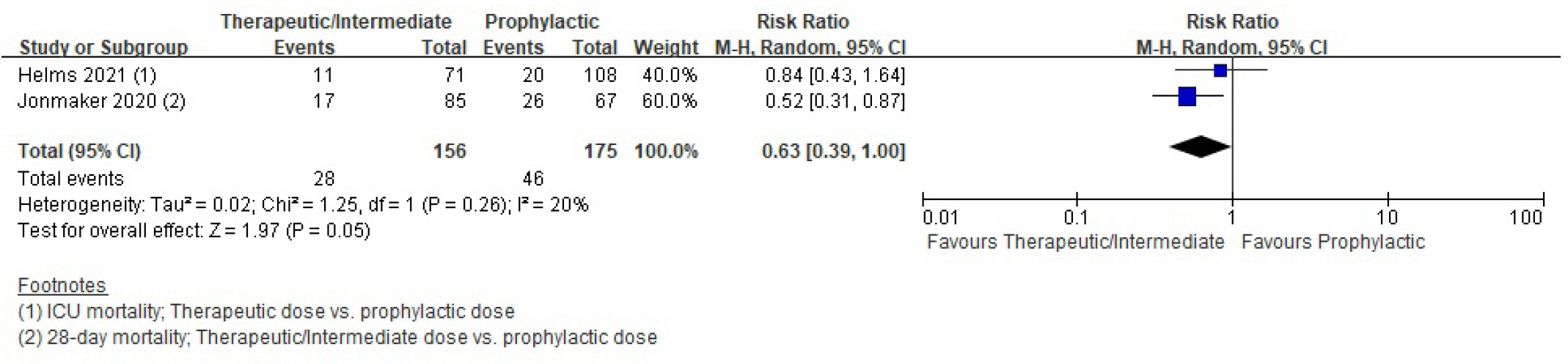
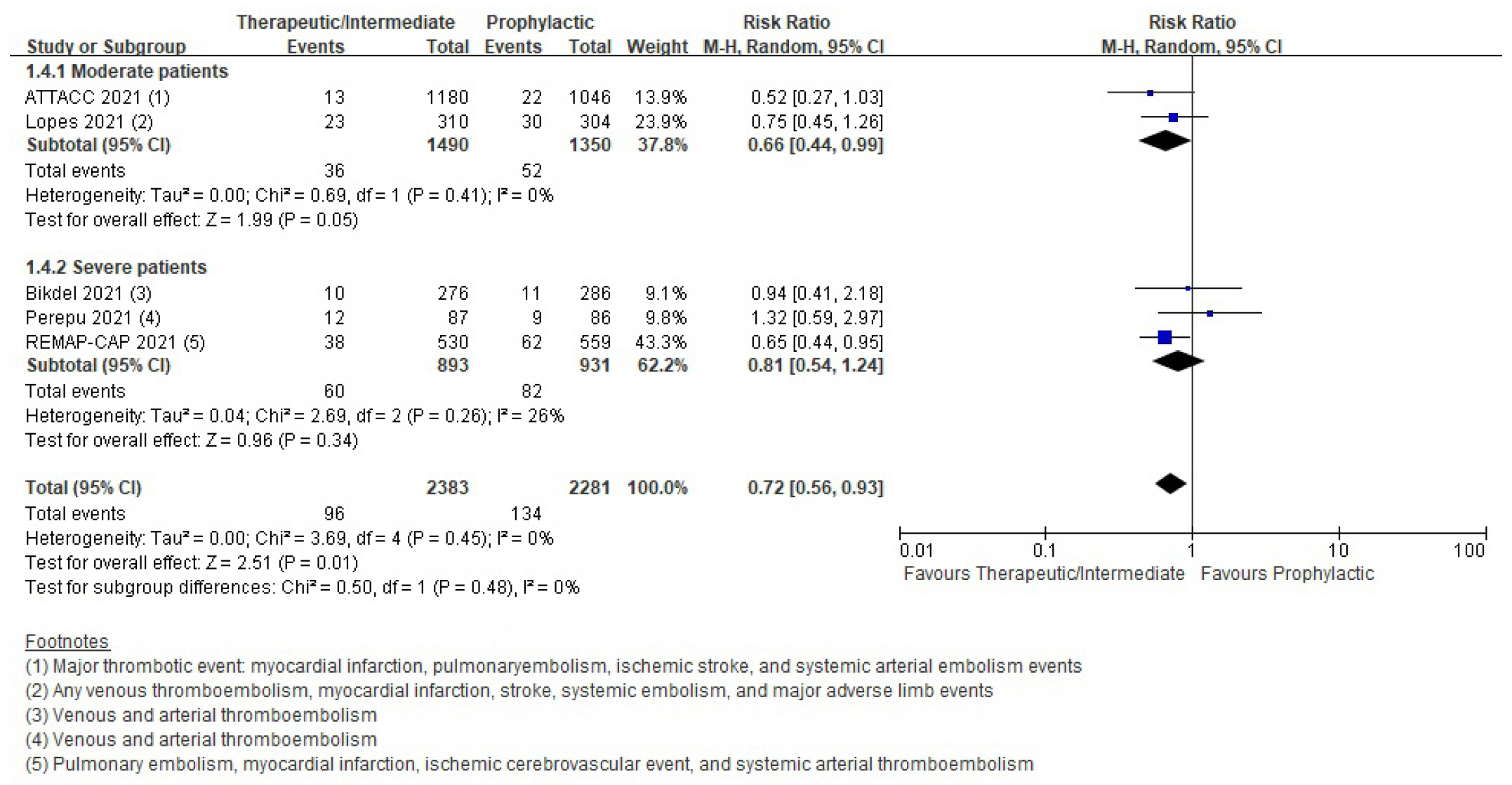
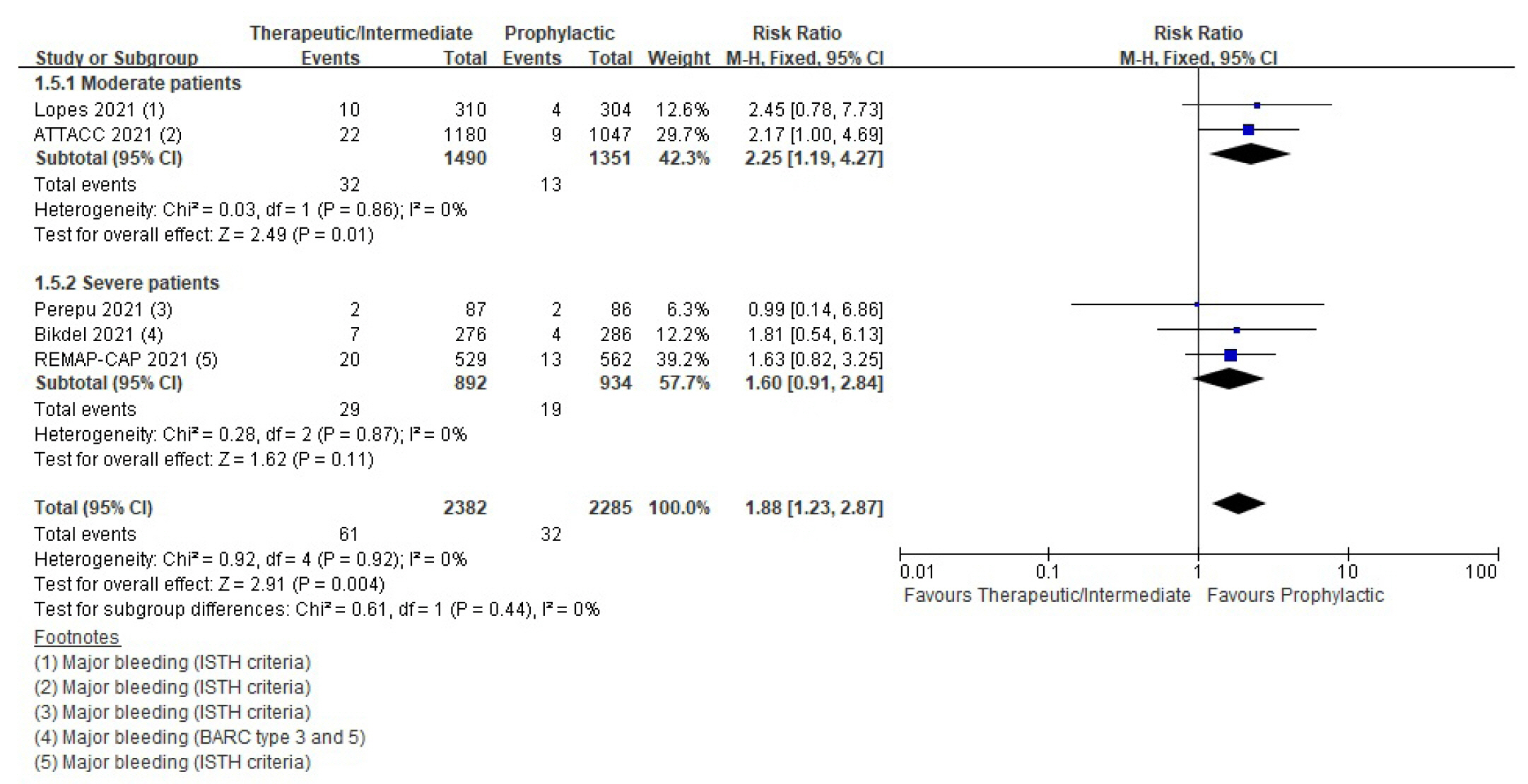
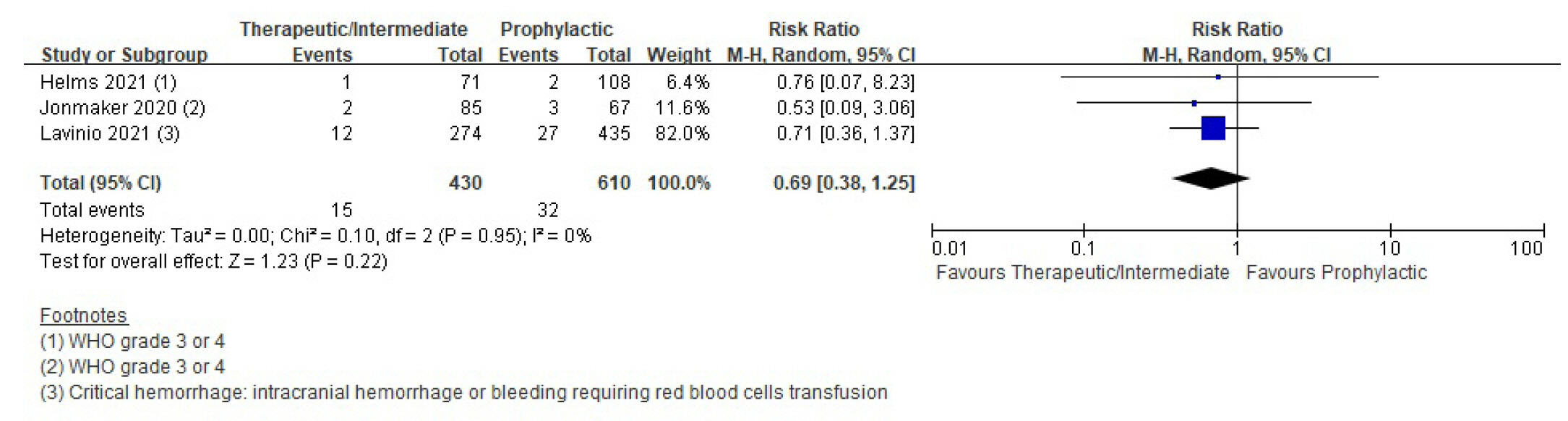
Patients with coronavirus disease 2019 (COVID-19) infections often have macrovascular or microvascular thrombosis and inflammation, which are known to be associated with a poor prognosis. Heparin has been hypothesized that administration of heparin with treatment dose rather than prophylactic dose for prevention of deep vein thrombosis in COVID-19 patients. Methods: Studies comparing therapeutic or intermediate anticoagulation with prophylactic anticoagulation in COVID-19 patients were eligible. Mortality, thromboembolic events, and bleeding were the primary outcomes. PubMed, Embase, the Cochrane Library, and KMbase were searched up to July 2021. A meta-analysis was performed using random-effect model. Subgroup analysis was conducted according to disease severity. Results: Six randomized controlled trials (RCTs) with 4,678 patients and four cohort studies with 1,080 patients were included in this review. In the RCTs, the therapeutic or intermediate anticoagulation was associated with significant reductions in the occurrence of thromboembolic events (5 studies, n=4,664; relative risk [RR], 0.72; P=0.01), and a significant increase in bleeding events (5 studies, n=4,667; RR, 1.88; P=0.004). In the moderate patients, therapeutic or intermediate anticoagulation was more beneficial than prophylactic anticoagulation in terms of thromboembolic events, but showed significantly higher bleeding events. In the severe patients, the incidence of thromboembolic and bleeding events in the therapeutic or intermediate. Conclusions: The study findings suggest that prophylactic anticoagulant treatment should be used in patients with moderate and severe COVID-19 infection groups. Further studies are needed to determine more individualized anticoagulation guidance for all COVID-19 patients.
Keyword
Figure
Reference
-
1. Godoy LC, Goligher EC, Lawler PR, Slutsky AS, Zarychanski R. Anticipating and managing coagulopathy and thrombotic manifestations of severe COVID-19. CMAJ. 2020; 192:E1156–61.
Article2. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020; 324:799–801.
Article3. McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020; 2:e437–45.
Article4. Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020; 18:1743–6.
Article5. Miesbach W, Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020; 26:1076029620938149.
Article6. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020; 135:2033–40.
Article7. Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, Painter CD, et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020; 183:1043–57.
Article8. Buijsers B, Yanginlar C, Maciej-Hulme ML, de Mast Q, van der Vlag J. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine. 2020; 59:102969.
Article9. Mycroft-West CJ, Su D, Pagani I, Rudd TR, Elli S, Gandhi NS, et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the spike S1 receptor-binding domain with heparin. Thromb Haemost. 2020; 120:1700–15.
Article10. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021; 372:n160.
Article11. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019; 10:ED000142.
Article12. Kim SY, Seo HJ, Lee YJ, Park JE. Study design algorithm for medical literature of intervention (DAMI) and Risk of Bias for Nonrandomized Studies (RoBANS) ver 2.0 by HIRA. Health Insurance Review and Assessment Service;2013.13. Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66:408–14.
Article14. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64:401–6.
Article15. ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators, Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021; 385:790–802.
Article16. Bikdeli B, Talasaz AH, Rashidi F, Bakhshandeh H, Rafiee F, Rezaeifar P, et al. Intermediate-dose versus standard-dose prophylactic anticoagulation in patients with COVID-19 admitted to the intensive care unit: 90-day results from the INSPIRATION randomized trial. Thromb Haemost. 2022; 122:131–41.
Article17. Lopes RD, de Barros E Silva PG, Furtado RH, Macedo AV, Bronhara B, Damiani LP, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021; 397:2253–63.18. Perepu US, Chambers I, Wahab A, Ten Eyck P, Wu C, Dayal S, et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. J Thromb Haemost. 2021; 19:2225–34.
Article19. REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators, Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021; 385:777–89.
Article20. INSPIRATION Investigators, Sadeghipour P, Talasaz AH, Rashidi F, Sharif-Kashani B, Beigmohammadi MT, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021; 325:1620–30.21. Helms J, Severac F, Merdji H, Schenck M, Clere-Jehl R, Baldacini M, et al. Higher anticoagulation targets and risk of thrombotic events in severe COVID-19 patients: bi-center cohort study. Ann Intensive Care. 2021; 11:14.
Article22. Jonmarker S, Hollenberg J, Dahlberg M, Stackelberg O, Litorell J, Everhov ÅH, et al. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Crit Care. 2020; 24:653.
Article23. Lavinio A, Ercole A, Battaglini D, Magnoni S, Badenes R, Taccone FS, et al. Safety profile of enhanced thromboprophylaxis strategies for critically ill COVID-19 patients during the first wave of the pandemic: observational report from 28 European intensive care units. Crit Care. 2021; 25:155.
Article24. Taccone FS, Gevenois PA, Peluso L, Pletchette Z, Lheureux O, Brasseur A, et al. Higher intensity thromboprophylaxis regimens and pulmonary embolism in critically ill coronavirus disease 2019 patients. Crit Care Med. 2020; 48:e1087–90.
Article25. Vincent JL, Levi M, Hunt BJ. Prevention and management of thrombosis in hospitalised patients with COVID-19 pneumonia. Lancet Respir Med. 2022; 10:214–20.
Article26. Poor HD. Pulmonary thrombosis and thromboembolism in COVID-19. Chest. 2021; 160:1471–80.
Article27. Ma Z, Yang KY, Huang Y, Lui KO. Endothelial contribution to COVID-19: an update on mechanisms and therapeutic implications. J Mol Cell Cardiol. 2022; 164:69–82.
Article28. Phung OJ, Kahn SR, Cook DJ, Murad MH. Dosing frequency of unfractionated heparin thromboprophylaxis: a meta-analysis. Chest. 2011; 140:374–81.
Article29. Kleber FX, Witt C, Vogel G, Koppenhagen K, Schomaker U, Flosbach CW, et al. Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease. Am Heart J. 2003; 145:614–21.
Article30. Bergmann JF, Neuhart E. A multicenter randomized double-blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in-patients bedridden for an acute medical illness. Thromb Haemost. 1996; 76:529–34.
Article31. Forette B, Wolmark Y. Calcium nadroparin in the prevention of thromboembolic disease in elderly subjects: study of tolerance. Presse Med. 1995; 24:567–71.32. Cohen AT, Spiro TE, Büller HR, Haskell L, Hu D, Hull R, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013; 368:513–23.
Article33. Cohen AT, Harrington RA, Goldhaber SZ, Hull RD, Wiens BL, Gold A, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016; 375:534–44.
Article34. Decousus H, Tapson VF, Bergmann JF, Chong BH, Froehlich JB, Kakkar AK, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011; 139:69–79.
Article35. Schulman S, Sholzberg M, Spyropoulos AC, Zarychanski R, Resnick HE, Bradbury CA, et al. ISTH guidelines for antithrombotic treatment in COVID-19. J Thromb Haemost. 2022; 20:2214–25.
Article36. Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020; 18:1859–65.
Article37. Cuker A, Tseng EK, Nieuwlaat R, Angchaisuksiri P, Blair C, Dane K, et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: January 2022 update on the use of therapeutic-intensity anticoagulation in acutely ill patients. Blood Adv. 2022; 6:4915–23.
Article38. Panel CT. Coronavirus disease 2019 (COVID-19) treatment guidelines [Internet]. National Institutes of Health;2023. [cited 2023 Feb 1]. Available from: https://www.covid19treatmentguidelines.nih.gov/.39. National Evidence-based Healthcare Collaborating Agency (NECA). Korean COVID-19 living guideline: anticoagulant recommendations [Internet]. NECA;2022. [cited 2023 Feb 1]. Available from: https://www.neca.re.kr/lay1/bbs/S1T11C174/F/58/view.do?article_seq=9067&cpage=1&rows=10000&condition=&keyword=&show=&cat.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of safety and efficacy between therapeutic or intermediate versus prophylactic anticoagulation for thrombosis in COVID-19 patients: a systematic review and meta-analysis
- Comment on “Comparison of efficacy and safety between palonosetron and ondansetron to prevent postoperative nausea and vomiting in patients undergoing laparoscopic surgery: a systematic review and meta-analysis”
- The Prevalence of Post-Traumatic Stress Disorder in the General Population during the COVID-19 Pandemic: A Systematic Review and Single-Arm Meta-Analysis
- Predictors of Mortality in Patients with COVID-19: A Systematic Review and Meta-analysis
- Depression in pregnant and postpartum women during COVID-19 pandemic: systematic review and meta-analysis