J Korean Neurosurg Soc.
2023 Jul;66(4):393-399. 10.3340/jkns.2022.0144.
Chiari Malformation with Surgically Induced Open Neural Tube Defect in Late Chick Embryos : Characterization by Magnetic Resonance Imaging and Histopathological Analysis
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Division of Pediatric Neurosurgery, Seoul National University Children’s Hospital, Seoul, Korea
- 3Department of Neurosurgery, Jeju National University Hospital, Jeju National University School of Medicine, Jeju, Korea
- KMID: 2543530
- DOI: http://doi.org/10.3340/jkns.2022.0144
Abstract
Objective
: Chiari II malformation (CM II) is still the main cause of severe morbidity and mortality in children with open neural tube defects (ONTDs). The goal of this study was to validate a CM II model in late-stage chick embryos with surgically induced ONTDs.
Methods
: To make the chick embryo model of ONTD, their neural tubes were opened for a length of 5–6 somites at the thoracic level in Hamburger and Hamilton stage 18 chick embryos (n=150). They were reincubated in ovo. up to a total age of 17–21 days. A total of 19 embryos survived and were assigned to either the postoperative day (POD) 14–15 group (n=6) or the POD 17–18 group (n=13). Magnetic resonance imaging (MRI) and histopathologic findings of embryo heads with spinal ONTDs were compared with age-matched normal chick embryos.
Results
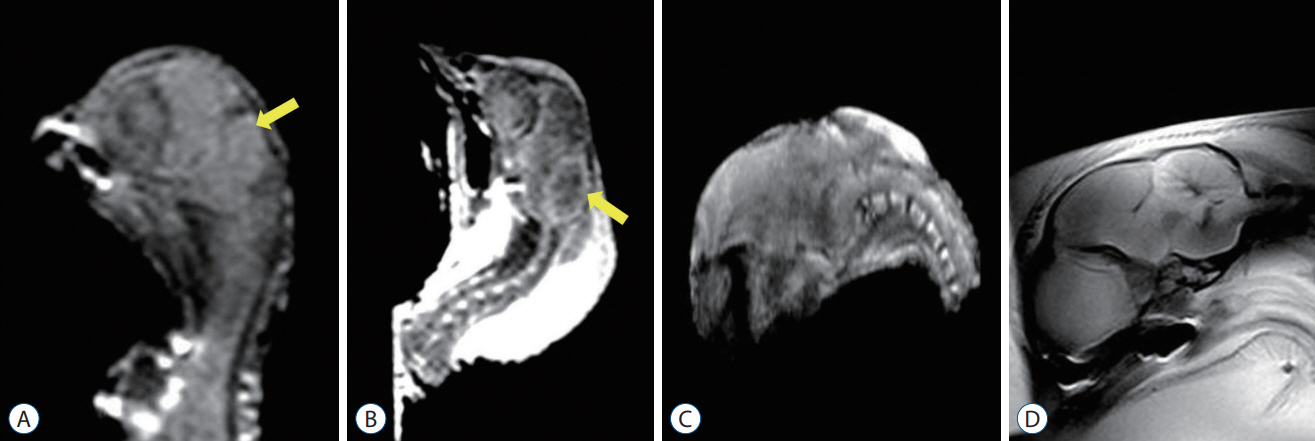
: The chick embryos with ONTDs demonstrated definite and constant structural changes, such as downward displacement of the cerebellum to just above the foramen magnum and narrow and small cerebrospinal fluid spaces in the crowded small posterior fossa. These morphologic features were more prominent in the POD 17–18 group than in the POD 14–15 group.
Conclusion
: This is the first description of CM II with spinal ONTD in a late-stage chick embryo model with MRI and histopathological analysis. The morphological changes of the posterior fossa in this study mimic those of CM II associated with spinal ONTD in humans. This model will facilitate investigation of the pathogenesis of CM II.
Keyword
Figure
Cited by 1 articles
-
Editor’s Pick in July 2023
Bum-Tae Kim
J Korean Neurosurg Soc. 2023;66(4):341-343. doi: 10.3340/jkns.2023.0117.
Reference
-
References
1. Bouchard S, Davey MG, Rintoul NE, Walsh DS, Rorke LB, Adzick NS. Correction of hindbrain herniation and anatomy of the vermis after in utero repair of myelomeningocele in sheep. J Pediatr Surg. 38:451–458. discussion 451-458. 2003.
Article2. Briner W, Lieske R. Arnold-Chiari-like malformation associated with a valproate model of spina bifida in the rat. Teratology. 52:306–311. 1995.
Article3. Fontecha CG, Peiro JL, Sevilla JJ, Aguirre M, Soldado F, Fresno L, et al. Fetoscopic coverage of experimental myelomeningocele in sheep using a patch with surgical sealant. Eur J Obstet Gynecol Reprod Biol. 156:171–176. 2011.
Article4. Glenn OA. MR imaging of the fetal brain. Pediatr Radiol. 40:68–81. 2010.
Article5. Griffiths PD, Wilkinson ID, Variend S, Jones A, Paley MN, Whitby E. Differential growth rates of the cerebellum and posterior fossa assessed by post mortem magnetic resonance imaging of the fetus: implications for the pathogenesis of the chiari 2 deformity. Acta Radiol. 45:236–242. 2004.
Article6. Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 88:49–92. 1951.
Article7. Inagaki T, Schoenwolf GC, Walker ML. Experimental model: change in the posterior fossa with surgically induced spina bifida aperta in mouse. Pediatr Neurosurg. 26:185–189. 1997.
Article8. McLone DG, Knepper PA. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci. 15:1–12. 1989.
Article9. Paek BW, Farmer DL, Wilkinson CC, Albanese CT, Peacock W, Harrison MR, et al. Hindbrain herniation develops in surgically created myelomeningocele but is absent after repair in fetal lambs. Am J Obstet Gynecol. 183:1119–1123. 2000.
Article10. Park TS, Hoffman HJ, Hendrick EB, Humphreys RP. Experience with surgical decompression of the Arnold-Chiari malformation in young infants with myelomeningocele. Neurosurgery. 13:147–152. 1983.
Article11. Sim KB, Cho BK, Chi JG, Wang KC. Morphological study of surgically induced open neural tube defect in old (14 and 21 days) chick embryos. Neurosci Lett. 192:61–64. 1995.
Article12. Sim KB, Hong SK, Cho BK, Choi DY, Wang KC. Experimentally induced Chiari-like malformation with myeloschisis in chick embryos. J Korean Med Sci. 11:509–516. 1996.
Article13. Sim KB, Lee CS, Shin TK. A study for normal development of the posterior cranial fossa in the chick embryos (gestation 14-20 days) with MR images and histopathology. Environmental Mutagens and Carcinogens. 25:25–31. 2005.14. Weber Guimarães Barreto M, Ferro MM, Guimarães Bittencourt D, Violin Pereira LA, Barini R, Sbragia L. Arnold-Chiari in a fetal rat model of dysraphism. Fetal Diagn Ther. 20:437–441. 2005.
Article15. Williams H. A unifying hypothesis for hydrocephalus, Chiari malformation, syringomyelia, anencephaly and spina bifida. Cerebrospinal Fluid Res. 5:7. 2008.
Article16. Wright C, Sibley CP, Baker PN. The role of fetal magnetic resonance imaging. Arch Dis Child Fetal Neonatal Ed. 95:F137–141. 2010.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Re-closure Capacity of Surgically Induced Open Neural Tube Defect in Chick Embryos
- Experimentally induced Chiari-like malformation with myeloschisis in chick embryos
- Re-closure by the Skin Graft of the Surgically Induced Spinal Open Neural Tube Defect in Chick Embryos
- Neural Tube Defect of Chick Embryos by Needle Puncture and Albumen Removal
- The Effect of Ca++ on Neurulation of Early Chick Embryos