Korean J Gastroenterol.
2023 May;81(5):209-215. 10.4166/kjg.2022.139.
Development of a New Liquid Type Rapid Urease Test Kit (Helicotest ® ): Comparison with Other Commercial Kits
- Affiliations
-
- 1Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- 2Department of Otolaryngology‑Head & Neck Surgery, Gachon University Gil Medical Center, Incheon, Korea
- KMID: 2542787
- DOI: http://doi.org/10.4166/kjg.2022.139
Abstract
- Background/Aims
A quick and accurate diagnosis of Helicobacter pylori (H. pylori) infections is vital for effectively managing many upper gastrointestinal tract diseases. Many diagnostic methods have been developed for rapid and accurate diagnosis, including invasive and non-invasive methods, but each tool has some limitations. Among the invasive diagnostic methods, the rapid urease test (RUT) is a relatively time-saving and accurate method, but a variation in the reaction time range causes inconvenience and inefficiency in the clinical field. This study developed a liquid-type medium, Helicotest ® , to enable faster detection. This study examined the reaction time of a new liquid-type RUT kit with other commercial kits.
Methods
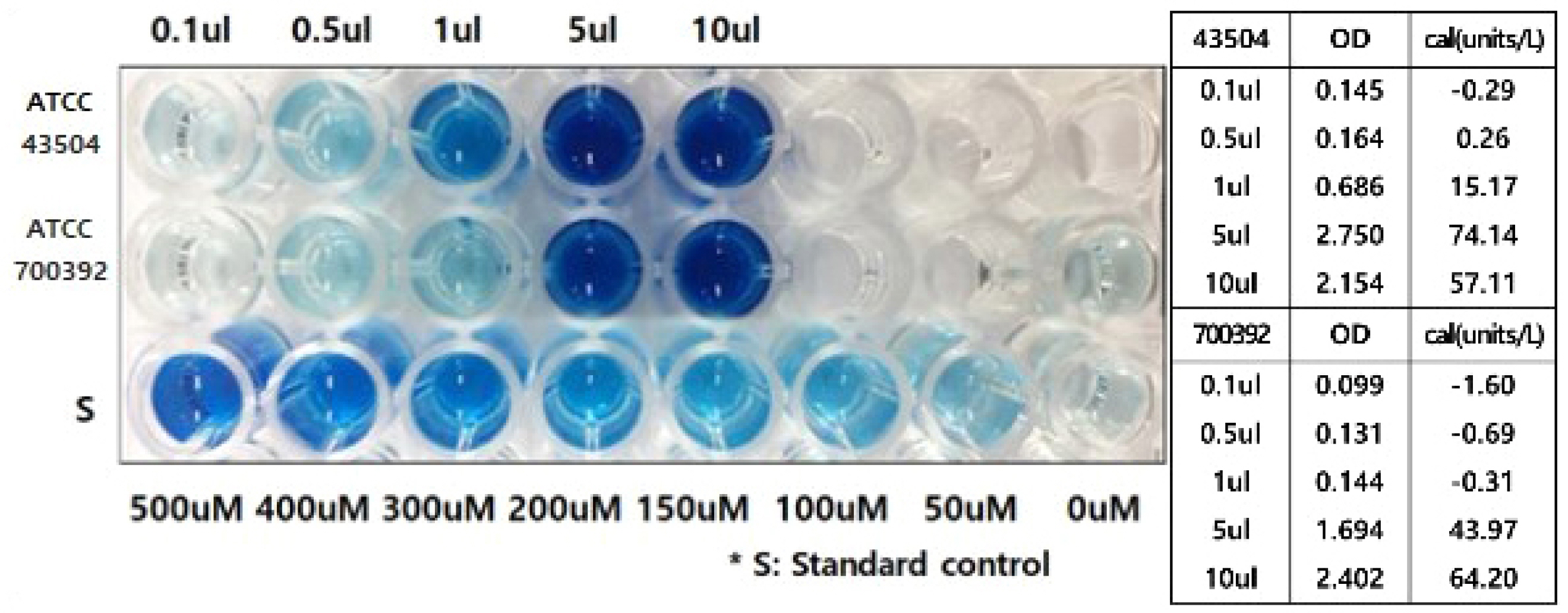
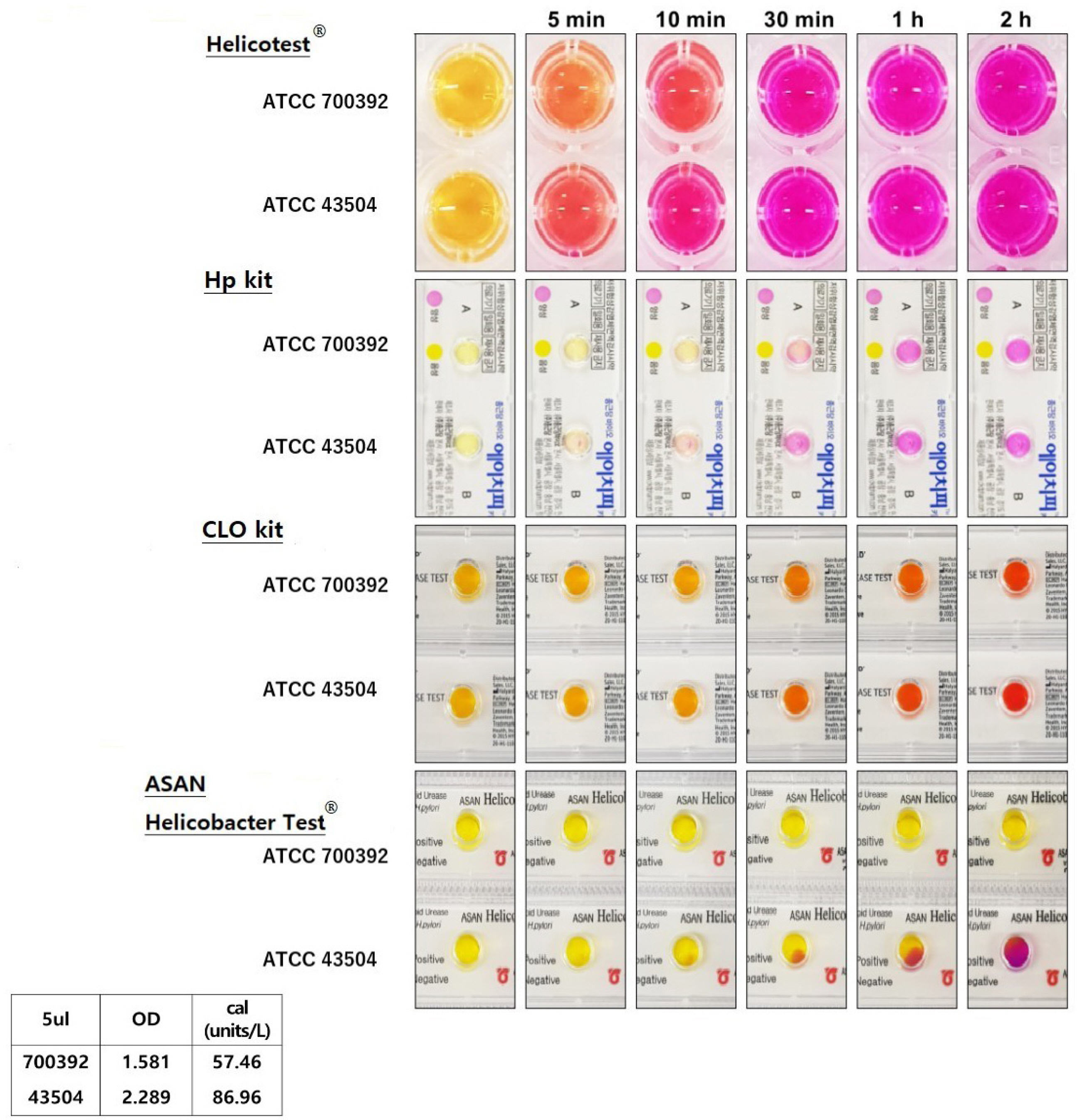
Two H. pylori strains were cultured (H. pylori ATCC 700392 and 43504), and the urease activity of H. pylori was measured using a urease activity assay kit (MAK120, Sigma Aldrich). Four RUT kits were used to compare the time of H. pylori detection, including Helicotest ® (Won Medical, Bucheon, Korea), Hp kit (Chong Kun Dang, Seoul, Korea), CLO kit (Halyard, Alpharetta, GA, USA), and ASAN Helicobacter Test ® (ASAN, Seoul, Korea).
Results
The detection of H. pylori was possible in bacterial amounts less than 10 μL. The color change was detected from five minutes with bacterial densities of 5 μL and 10 μL for both strains, whereas 30 minutes and one hour were required for 0.5 μL and a 1 μL bacterial density of ATCC 43504 and 700392 strains, respectively.
Conclusions
Compared to other RUT kits, Helicotest ® showed the fastest reaction. Therefore, faster diagnosis in clinical practice is expected.
Keyword
Figure
Reference
-
1. Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. 2007; American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 102:1808–1825. DOI: 10.1111/j.1572-0241.2007.01393.x. PMID: 17608775.
Article2. Choi IJ, Kook MC, Kim YI, et al. 2018; Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 378:1085–1095. DOI: 10.1056/NEJMoa1708423. PMID: 29562147.
Article3. Choi IJ, Kim CG, Lee JY, et al. 2020; Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 382:427–436. DOI: 10.1056/NEJMoa1909666. PMID: 31995688.
Article4. Choi YI, Chung JW. 2020; Helicobacter pylori eradication in patients undergoing gastrectomy: diagnosis and therapy. Korean J Helicobacter Up Gastrointest Res. 20:204–209. DOI: 10.7704/kjhugr.2019.0037.
Article5. Lee YC, Chiang TH, Chou CK, et al. 2016; Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 150:1113–1124.e5. DOI: 10.1053/j.gastro.2016.01.028. PMID: 26836587.
Article6. Kim YH, Shin SW. 2018; Helicobacter pylori and prevention of gastric cancer. N Engl J Med. 378:2244. DOI: 10.1056/NEJMc1805129.7. Tseng CA, Wang WM, Wu DC. 2005; Comparison of the clinical feasibility of three rapid urease tests in the diagnosis of Helicobacter pylori infection. Dig Dis Sci. 50:449–452. DOI: 10.1007/s10620-005-2456-5. PMID: 15810624.8. Pohl D, Keller PM, Bordier V, Wagner K. 2019; Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J Gastroenterol. 25:4629–4660. DOI: 10.3748/wjg.v25.i32.4629. PMID: 31528091. PMCID: PMC6718044.9. Monteiro L, Mégraud F. 1999; [How to detect Helicobacter pylori before and after eradication treatment?]. Gastroenterol Clin Biol. 23(10 Pt 2):C3–19. French.10. McNicholl AG, Ducons J, Barrio J, et al. Helicobacter pylori Study Group of the Asociación Española de Gastroenterología (AEG). Accuracy of the ultra-rapid urease test for diagnosis of Helicobacter pylori infection. Gastroenterol Hepatol. 2017; 40:651–657. English, Spanish. DOI: 10.1016/j.gastre.2017.07.012.11. Uotani T, Graham DY. 2015; Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med. 3:9.12. Malfertheiner P, Megraud F, O'Morain CA, et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017; 66:6–30. DOI: 10.1136/gutjnl-2016-312288. PMID: 27707777.
Article13. Wang YK, Kuo FC, Liu CJ, et al. 2015; Diagnosis of Helicobacter pylori infection: current options and developments. World J Gastroenterol. 21:11221–11235. DOI: 10.3748/wjg.v21.i40.11221. PMID: 26523098. PMCID: PMC4616200.
Article14. Yousfi MM, El-Zimaity HM, Cole RA, Genta RM, Graham DY. 1997; Comparison of agar gel (CLOtest) or reagent strip (PyloriTek) rapid urease tests for detection of Helicobacter pylori infection. Am J Gastroenterol. 92:997–999.15. Vaira D, Vakil N, Gatta L, et al. 2010; Accuracy of a new ultrafast rapid urease test to diagnose Helicobacter pylori infection in 1000 consecutive dyspeptic patients. Aliment Pharmacol Ther. 31:331–338. DOI: 10.1111/j.1365-2036.2009.04196.x. PMID: 19891666.16. Hong SJ, Ryu CB, Kim JO, et al. 2001; Diagnostic usefulness of Hp kit test for the detection of Helicobacter pylori infection. Korean J Gastrointest Endosc. 22:8–13.17. Roe IH, Lee MI, Kim JT, et al. 1999; Comparison of Hp kit test and CLO test for the diagnosis of Helicobacter pylori infection. Korean J Gastroenterol. 34:448–454.18. Laine L, Lewin D, Naritoku W, Estrada R, Cohen H. 1996; Prospective comparison of commercially available rapid urease tests for the diagnosis of Helicobacter pylori. Gastrointest Endosc. 44:523–526. DOI: 10.1016/S0016-5107(96)70002-0. PMID: 8934155.19. Lee J, Kim PS, Lee K, Lee JH, Lee JK, Lee CH. 2003; Evaluation of Asan Helicobacter test for diagnosis of Helicobacter pylori Infection. Korean J Clin Microbiol. 6:156–159.20. Kinoshita-Daitoku R, Ogura Y, Kiga K, et al. 2020; Complete genome sequence of Helicobacter pylori Strain ATCC 43504, a type strain that can infect gerbils. Microbiol Resour Announc. 9:e00105–20. DOI: 10.1128/MRA.00105-20. PMID: 32354967. PMCID: PMC7193922.
Article21. Cerda O, Rivas A, Toledo H. 2003; Helicobacter pylori strain ATCC700392 encodes a methyl-accepting chemotaxis receptor protein (MCP) for arginine and sodium bicarbonate. FEMS Microbiol Lett. 224:175–181. DOI: 10.1016/S0378-1097(03)00423-3. PMID: 12892880.
Article22. Jung HK, Kang SJ, Lee YC, et al. Korean College of Helicobacter and Upper Gastrointesinal Research. Evidence based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Korean J Intern Med. 2021; 36:807–838. DOI: 10.3904/kjim.2020.701. PMID: 34092054. PMCID: PMC8273819.23. Lee JH, Ahn JY, Choi KD, et al. Korean College of Helicobacter; Upper Gastrointestinal Research. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: A prospective multicenter study. Helicobacter. 2019; 24:e12592. DOI: 10.1111/hel.12592. PMID: 31111572.24. Lee JW, Kim N, Kim JM, et al. 2013; Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 18:206–214. DOI: 10.1111/hel.12031. PMID: 23241101.
Article25. Lee JY, Kim N, Nam RH, Choi SI, Lee JW, Lee DH. 2019; Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter. 24:e12660. DOI: 10.1111/hel.12660. PMCID: PMC6790945.
Article26. Wenzhen Y, Yumin L, Quanlin G, et al. 2010; Is antimicrobial susceptibility testing necessary before first-line treatment for Helicobacter pylori infection? Meta-analysis of randomized controlled trials. Intern Med. 49:1103–1109. DOI: 10.2169/internalmedicine.49.3031. PMID: 20558925.
Article27. López-Góngora S, Puig I, Calvet X, et al. 2015; Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 70:2447–2455. DOI: 10.1093/jac/dkv155. PMID: 26078393.
Article28. Graham DY, Lew GM, Malaty HM, et al. 1992; Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology. 102:493–496. DOI: 10.1016/0016-5085(92)90095-G. PMID: 1732120.
Article29. Graham DY, Opekun AR, Hammoud F, et al. 2003; Studies regarding the mechanism of false negative urea breath tests with proton pump inhibitors. Am J Gastroenterol. 98:1005–1009. DOI: 10.1111/j.1572-0241.2003.07426.x. PMID: 12809820.
Article30. Chey WD, Chathadi KV, Montague J, Ahmed F, Murthy U. 2001; Intragastric acidification reduces the occurrence of false-negative urea breath test results in patients taking a proton pump inhibitor. Am J Gastroenterol. 96:1028–1032. DOI: 10.1111/j.1572-0241.2001.03687.x. PMID: 11316142.
Article31. Forné M, Viver JM, Espinós JC, Coll I, Tresserra F, Garau J. 1995; Impact of colloidal bismuth subnitrate in the eradication rates of Helicobacter pylori infection-associated duodenal ulcer using a short treatment regimen with omeprazole and clarithromycin: a randomized study. Am J Gastroenterol. 90:718–721.32. Vaira D, Holton J, Cairns S, et al. 1988; Urease tests for Campylobacter pylori: care in interpretation. J Clin Pathol. 41:812–813. DOI: 10.1136/jcp.41.7.812. PMID: 3410977. PMCID: PMC1141596.
Article33. Attumi TA, Graham DY. 2011; Follow-up testing after treatment of Helicobacter pylori infections: cautions, caveats, and recommendations. Clin Gastroenterol Hepatol. 9:373–375. DOI: 10.1016/j.cgh.2010.12.025. PMID: 21195791.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Usefulness of Helicobacter pylori IgG Ab Assay: Comparison of Six Commercial Kits
- Comparison of RNA Extraction Kits for Detection of HCV RNA
- Comparison of Hp Kit Test and CLO Test for the Diagnosis of Helicobacter pylori Infection
- Diagnostic Usefulness of Hp Kit Test for the Detection of Helicobacter pylori Infection
- An Alternative Method for a Rapid Urease Test Using Back-table Gastric Mucosal Biopsies from Gastrectomy Specimen for Making the Diagnosis of Helicobacter pylori Infection in Patients with Gastric Cancer