Korean J Gastroenterol.
2023 May;81(5):185-188. 10.4166/kjg.2023.042.
Approach to the Patients with Elevated CA 19-9
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- KMID: 2542783
- DOI: http://doi.org/10.4166/kjg.2023.042
Abstract
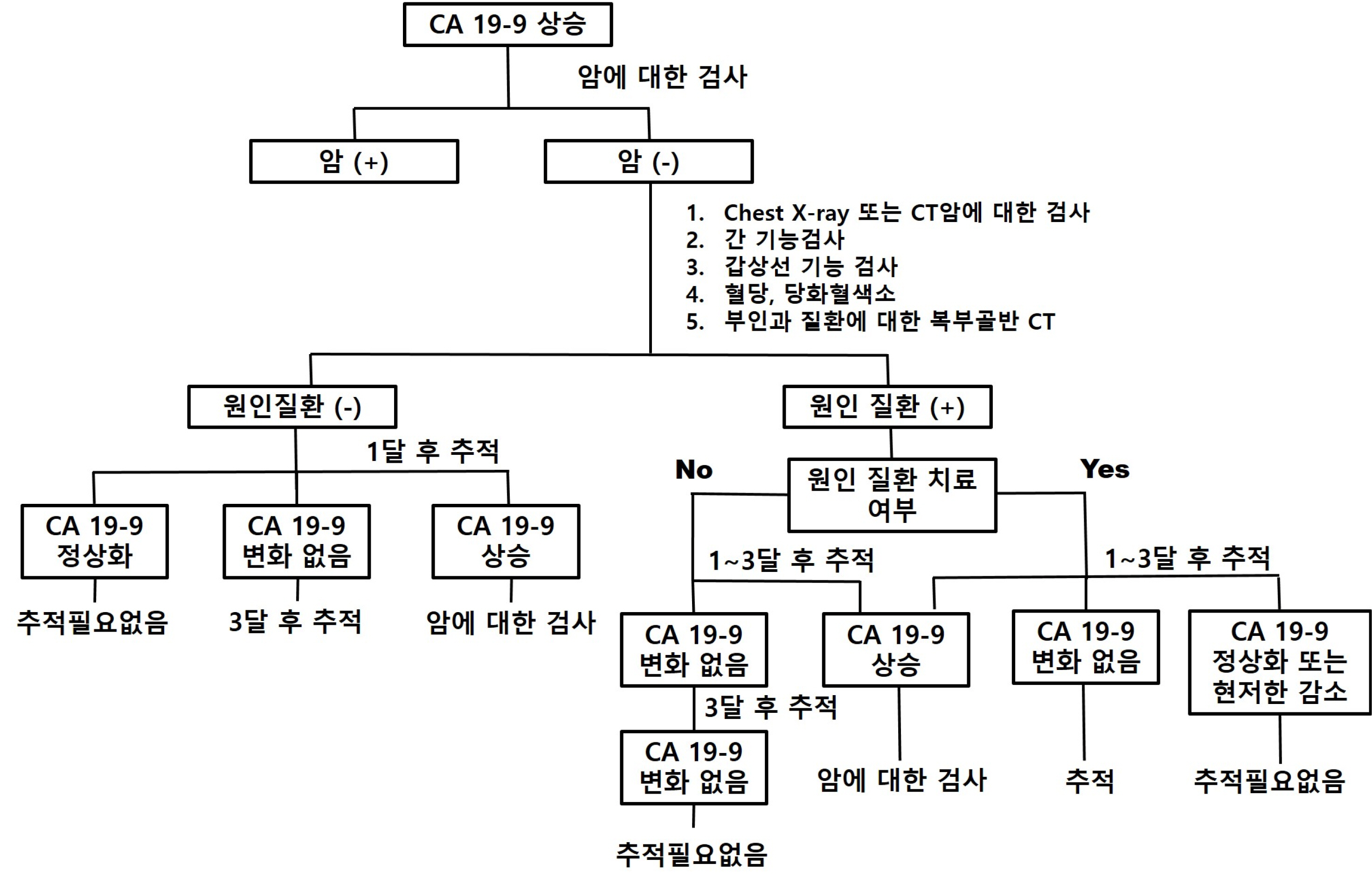
- With the widespread adoption of health check-ups, tumor markers are being used for screening healthy individuals without symptoms related to cancer. Although CA 19-9 is known to have diagnostic value when a patient presents with symptoms, the evidence for its clinical value as a cancer screening test in asymptomatic patients is still lacking. However, patients who experience an increase in CA 19-9 levels may feel anxious about the possibility of having cancer and may seek medical attention. If the CA 19-9 level is elevated, it may be necessary to consider initial testing for malignant tumors of the pancreas. It should be recognized that the level can also increase in malignant tumors of the gastrointestinal tract, thyroid, and reproductive organs. Since the CA 19-9 levels can also increase in various benign diseases, it is important to evaluate if there is an underlying benign disease through appropriate testing and follow-up to reduce patient anxiety and discontinue unnecessary follow-up tests.
Keyword
Figure
Reference
-
1. Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V. 2006; Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology. 70:255–264. DOI: 10.1159/000094888. PMID: 16899980.
Article2. Safi F, Roscher R, Bittner R, Schenkluhn B, Dopfer HP, Beger HG. 1987; High sensitivity and specificity of CA 19-9 for pancreatic carcinoma in comparison to chronic pancreatitis. Serological and immunohistochemical findings. Pancreas. 2:398–403. DOI: 10.1097/00006676-198707000-00006. PMID: 3306667.
Article3. Kim BJ, Lee KT, Moon TG, et al. 2008; How do we interpret an elevated carbohydrate antigen 19-9 level in asymptomatic subjects? Gastroenterology. 134:A326–A327. DOI: 10.1016/S0016-5085(08)61522-X.
Article4. Kim JE, Lee KT, Lee JK, Paik SW, Rhee JC, Choi KW. 2004; Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 19:182–186. DOI: 10.1111/j.1440-1746.2004.03219.x. PMID: 14731128.
Article5. Galli C, Basso D, Plebani M. 2013; CA 19-9: handle with care. Clin Chem Lab Med. 51:1369–1383. DOI: 10.1515/cclm-2012-0744. PMID: 23370912.
Article6. Goonetilleke KS, Siriwardena AK. 2007; Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 33:266–270. DOI: 10.1016/j.ejso.2006.10.004. PMID: 17097848.
Article7. Parra JL, Kaplan S, Barkin JS. 2005; Elevated CA 19-9 caused by Hashimotós thyroiditis: review of the benign causes of increased CA 19-9 level. Dig Dis Sci. 50:694–695. DOI: 10.1007/s10620-005-2559-z. PMID: 15844704.
Article8. Duffy MJ, Sturgeon C, Lamerz R, et al. 2010; Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 21:441–447. DOI: 10.1093/annonc/mdp332. PMID: 19690057.
Article9. Ito S, Gejyo F. 1999; Elevation of serum CA19-9 levels in benign diseases. Intern Med. 38:840–841. DOI: 10.2169/internalmedicine.38.840. PMID: 10563741.
Article10. Chang CY, Huang SP, Chiu HM, Lee YC, Chen MF, Lin JT. 2006; Low efficacy of serum levels of CA 19-9 in prediction of malignant diseases in asymptomatic population in Taiwan. Hepatogastroenterology. 53:1–4.11. Malesci A, Montorsi M, Mariani A, et al. 1992; Clinical utility of the serum CA 19-9 test for diagnosing pancreatic carcinoma in symptomatic patients: a prospective study. Pancreas. 7:497–502. DOI: 10.1097/00006676-199207000-00012. PMID: 1641392.
Article12. Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. 2005; The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 50:1734–1740. DOI: 10.1007/s10620-005-2927-8. PMID: 16133981.
Article13. Blechacz B. 2017; Cholangiocarcinoma: Current knowledge and new developments. Gut Liver. 11:13–26. DOI: 10.5009/gnl15568. PMID: 27928095. PMCID: PMC5221857.
Article14. Tao LY, Cai L, He XD, Liu W, Qu Q. 2010; Comparison of serum tumor markers for intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Am Surg. 76:1210–1213. DOI: 10.1177/000313481007601119. PMID: 21140686.
Article15. Sachan A, Saluja SS, Nekarakanti PK, et al. 2020; Raised CA19-9 and CEA have prognostic relevance in gallbladder carcinoma. BMC Cancer. 20:826. DOI: 10.1186/s12885-020-07334-x. PMID: 32867709. PMCID: PMC7457344.
Article16. Acharya A, Markar SR, Matar M, Ni M, Hanna GB. 2017; Use of tumor markers in gastrointestinal cancers: Surgeon perceptions and cost-benefit trade-off analysis. Ann Surg Oncol. 24:1165–1173. DOI: 10.1245/s10434-016-5717-y. PMID: 28008574. PMCID: PMC5374165.
Article17. Song YX, Huang XZ, Gao P, et al. 2015; Clinicopathologic and prognostic value of serum carbohydrate antigen 19-9 in gastric cancer: A meta-analysis. Dis Markers. 2015:549843. DOI: 10.1155/2015/549843. PMID: 26576068. PMCID: PMC4631884.
Article18. Duffy MJ, van Dalen A, Haglund C, et al. 2003; Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer. 39:718–727. DOI: 10.1016/S0959-8049(02)00811-0. PMID: 12651195.19. Kim S, Park BK, Seo JH, et al. 2020; Carbohydrate antigen 19-9 elevation without evidence of malignant or pancreatobiliary diseases. Sci Rep. 10:8820. DOI: 10.1038/s41598-020-65720-8. PMID: 32483216. PMCID: PMC7264353.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Abnormally high level of CA-19-9 in a benign ovarian cyst
- Normalization of Elevated CA 19-9 Level after Treatment in a Patient with the Nodular Bronchiectatic Form of Mycobacterium abscessus Lung Disease
- Usefulness of Tumor Marker CA 242 in the Diagnosis of Pancreatic Cancer
- An Immunohistochemical Study of CA 125, CA 19-9, and CA 15-3 in Ovarian Epithelial Tumors
- Usefulness of Carbohydrate Antigen 19-9 Test in Healthy People and Necessity of Medical Follow-up in Individuals with Elevated Carbohydrate Antigen 19-9 Level