J Korean Med Sci.
2023 May;38(21):e187. 10.3346/jkms.2023.38.e187.
Decrease of Muscle Mass in Young Patients With Neuromuscular Disease: Assessment of Sarcopenia
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Pediatric Orthopaedic Surgery, Severance Children’s Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2542604
- DOI: http://doi.org/10.3346/jkms.2023.38.e187
Abstract
- Background
Sarcopenia can be associated with the disease etiologies other than degenerative processes, such as neurologic disease including cerebral palsy, myelomeningocele, or Duchenne muscular dystrophy, even in children. Although the relationship between neurologic disease and scoliosis or ambulatory function is known, the mediators affecting scoliosis or gait function in these patients are unclear, an example might be sarcopenia. This study aimed to assess the degree of sarcopenia in young patients with neurologic diseases using computed tomography (CT), and analyze the correlation between sarcopenia and scoliosis or ambulatory function.
Methods
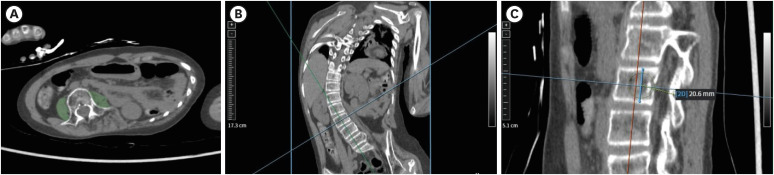
Pediatric and young adult patients (≤ 25 years old) who underwent whole-spine or lower-extremity CT were retrospectively included. From bilateral psoas muscle areas (PMAs) at the L3 level, the psoas muscle z-score (PMz) and psoas muscle index [PMI = PMA/(L3 height) 2 ] were calculated. The t-test, Fisher’s exact test, and logistic regression analyses were performed.
Results
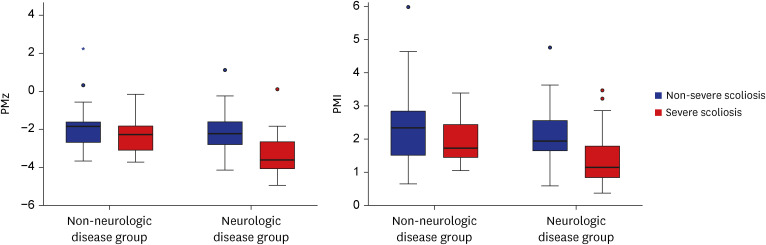
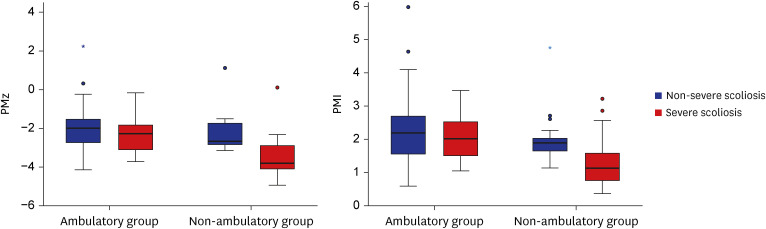
A total of 121 patients (56 men, mean age 12.2 ± 3.7 years) were included with 79 neurologic and 42 non-neurologic diseases. Patients with neurologic diseases had lower PMz (P = 0.013) and PMI (P = 0.026) than patients without. In neurologic disease patients, severe scoliosis patients showed lower PMz (P < 0.001) and PMI (P = 0.001). Non-ambulatory patients (n = 42) showed lower BMI (β = 0.727, P < 0.001) and PMz (β = 0.547, P = 0.025). In non-ambulatory patients, patients with severe scoliosis also showed lower PMz (P < 0.001) and PMI (P = 0.004).
Conclusion
Patients with neurologic diseases could have sarcopenia even in young age. Psoas muscle volume was also associated with ambulatory function in these patients. Sarcopenia was more severe in severe scoliosis patients in the non-ambulatory subgroup.
Keyword
Figure
Reference
-
1. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010; 39(4):412–423. PMID: 20392703.2. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48(4):601.3. Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol. 2015; 205(3):W255-66. PMID: 26102307.4. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008; 33(5):997–1006. PMID: 18923576.5. Jeon I, Bang MS, Lim JY, Shin HI, Leigh JH, Kim K, et al. Sarcopenia among adults with cerebral palsy in South Korea. PM R. 2019; 11(12):1296–1301. PMID: 30729753.6. Kerr Graham H, Selber P. Musculoskeletal aspects of cerebral palsy. J Bone Joint Surg Br. 2003; 85-B(2):157–166.7. Verschuren O, Smorenburg AR, Luiking Y, Bell K, Barber L, Peterson MD. Determinants of muscle preservation in individuals with cerebral palsy across the lifespan: a narrative review of the literature. J Cachexia Sarcopenia Muscle. 2018; 9(3):453–464. PMID: 29392922.8. Aftzoglou P. Sarcopenia and falls in patients with adult scoliosis. J Frailty Sarcopenia Falls. 2017; 2(4):83–87. PMID: 32300685.9. Kusumi K, Dunwoodie SL. The Genetics and Development of Scoliosis. Berlin: Springer;2009.10. Eguchi Y, Suzuki M, Yamanaka H, Tamai H, Kobayashi T, Orita S, et al. Associations between sarcopenia and degenerative lumbar scoliosis in older women. Scoliosis Spinal Disord. 2017; 12:9. PMID: 28331906.11. Eguchi Y, Toyoguchi T, Inage K, Fujimoto K, Orita S, Suzuki M, et al. Analysis of skeletal muscle mass in women over 40 with degenerative lumbar scoliosis. Eur Spine J. 2019; 28(7):1618–1625. PMID: 30515558.12. Park JS, Park YS, Kim J, Hur J, Choe DH. Sarcopenia and fatty degeneration of paraspinal muscle associated with increased sagittal vertical axis in the elderly: a cross-sectional study in 71 female patients. Eur Spine J. 2020; 29(6):1353–1361. PMID: 32322973.13. Alman B. Overview and comparison of idiopathic, neuromuscular, and congenital forms of scoliosis. The genetics and Development of Scoliosis. Berlin: Springer;2010. p. 73–79.14. Gilligan LA, Towbin AJ, Dillman JR, Somasundaram E, Trout AT. Quantification of skeletal muscle mass: sarcopenia as a marker of overall health in children and adults. Pediatr Radiol. 2020; 50(4):455–464. PMID: 31745597.15. Lurz E, Patel H, Lebovic G, Quammie C, Woolfson JP, Perez M, et al. Paediatric reference values for total psoas muscle area. J Cachexia Sarcopenia Muscle. 2020; 11(2):405–414. PMID: 31920002.16. Dohzono S, Sasaoka R, Takamatsu K, Nakamura H. Association between age and trunk muscle area and density in patients with spinal metastases. Asian Spine J. 2022; 16(5):677–683. PMID: 35654110.17. Sato K, Tominaga R, Endo T, Miura T, Iwabuchi M, Ito T, et al. Hip extensor strength influences dynamic postural changes during gait in patients with adult spinal deformity: a cross-sectional study using three-dimensional motion analysis. Asian Spine J. 2022; 16(5):643–650. PMID: 35184520.18. Yodoshi T, Orkin S, Arce Clachar AC, Bramlage K, Sun Q, Fei L, et al. Muscle mass is linked to liver disease severity in pediatric nonalcoholic fatty liver disease. J Pediatr. 2020; 223:93–99.e2. PMID: 32711755.19. Ritz A, Kolorz J, Hubertus J, Ley-Zaporozhan J, von Schweinitz D, Koletzko S, et al. Sarcopenia is a prognostic outcome marker in children with high-risk hepatoblastoma. Pediatr Blood Cancer. 2021; 68(5):e28862. PMID: 33438330.20. Boster JM, Browne LP, Pan Z, Zhou W, Ehrlich PF, Sundaram SS. Higher mortality in pediatric liver transplant candidates with sarcopenia. Liver Transpl. 2021; 27(6):808–817. PMID: 33621376.21. Jitwongwai S, Lertudomphonwanit C, Junhasavasdikul T, Fuangfa P, Tanpowpong P, Gesprasert G, et al. Low psoas muscle index as an unfavorable factor in children with end-stage liver disease undergoing liver transplantation. Pediatr Transplant. 2021; 25(5):e13996. PMID: 33734542.22. Verhagen MV, Levolger S, Hulshoff JB, Werner MJ, van der Doef HP, Viddeleer AR, et al. Utility of preoperative computed tomography-based body metrics in relation to postoperative complications in pediatric liver transplantation recipients. Liver Transpl. 2021; 27(12):1779–1787. PMID: 34118133.23. Kim H, Lee CK, Yeom JS, Lee JH, Cho JH, Shin SI, et al. Asymmetry of the cross-sectional area of paravertebral and psoas muscle in patients with degenerative scoliosis. Eur Spine J. 2013; 22(6):1332–1338. PMID: 23515711.24. Shortland A. Muscle deficits in cerebral palsy and early loss of mobility: can we learn something from our elders? Dev Med Child Neurol. 2009; 51(Suppl 4):59–63. PMID: 19740211.25. Multani I, Manji J, Tang MJ, Herzog W, Howard JJ, Graham HK. Sarcopenia, cerebral palsy, and botulinum toxin type A. JBJS Rev. 2019; 7(8):e4.26. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019; 43(3):241–249. PMID: 31311245.27. Beals RK. Nosologic and genetic aspects of scoliosis. Clin Orthop Relat Res. 1973; 93:23–32.28. Kim HS, Kwon JW, Park KB. Clinical issues in indication, correction, and outcomes of the surgery for neuromuscular scoliosis: narrative review in pedicle screw era. Neurospine. 2022; 19(1):177–187. PMID: 35130428.29. Igawa T, Ishii K, Isogai N, Suzuki A, Ishizaka M, Funao H. Prevalence of sarcopenia in idiopathic dropped head syndrome patients is similar to healthy volunteers. Sci Rep. 2021; 11(1):16213. PMID: 34376701.30. Hiyama A, Katoh H, Sakai D, Sato M, Tanaka M, Nukaga T, et al. Correlation analysis of sagittal alignment and skeletal muscle mass in patients with spinal degenerative disease. Sci Rep. 2018; 8(1):15492. PMID: 30341320.31. Eleswarapu A, O’Connor D, Rowan FA, Van Le H, Wick JB, Javidan Y, et al. Sarcopenia is an independent risk factor for proximal junctional disease following adult spinal deformity surgery. Global Spine J. 2022; 12(1):102–109. PMID: 32865046.32. Sukthankar A, Nerlich AG, Paesold G. Age-related changes of the spine. Spinal Disorders. Berlin: Springer;2008. p. 91–122.