Obstet Gynecol Sci.
2023 May;66(3):120-132. 10.5468/ogs.22261.
Preeclampsia and aspirin
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Kangwon National University College of Medicine, Chuncheon, Korea
- KMID: 2542219
- DOI: http://doi.org/10.5468/ogs.22261
Abstract
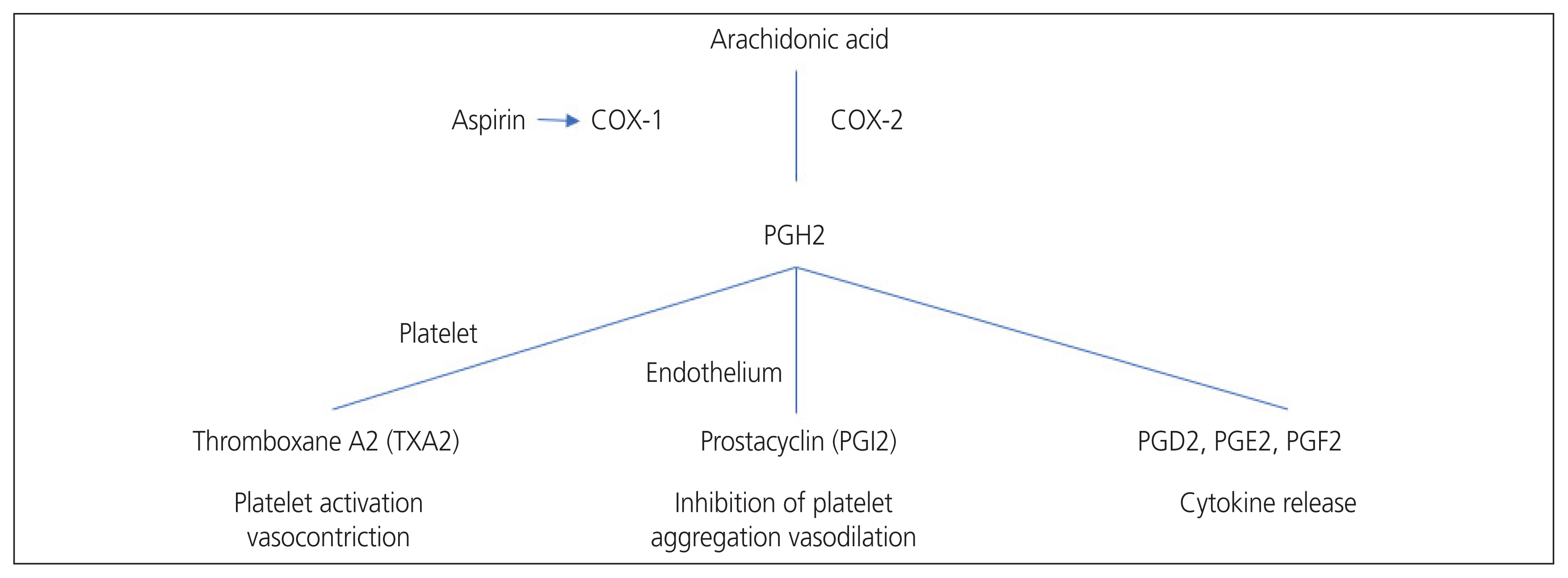
- Preeclampsia (PE) is a multisystem disorder that is an important cause of maternal and perinatal deaths. Currently, delivery is the only final treatment for PE. This practice is usually accompanied by premature birth, which inevitably increases neonatal morbidities. Aspirin is a non-selective non-steroidal anti-inflammatory drug that irreversibly inhibits cyclooxygenase enzymes involved in converting arachidonic acid to prostaglandins and thromboxane. Aspirin inhibits thromboxane A2 production via platelet aggregation, thereby increasing the prostacyclin/thromboxane A2 ratio and reducing platelet aggregation. Since the first case report of aspirin’s potential use during pregnancy was reported in 1978, many studies have attempted to confirm the effect of aspirin on PE, and the results have been controversial. However, this preventive strategy is generally accepted in clinical practice. As evidence for aspirin’s prevention of PE has been accumulating, a recent study investigated the effectiveness of aspirin at high doses of 150 mg, which is higher than before. However, there is an ongoing debate about how much aspirin should be used during pregnancy and when to start aspirin therapy. Guidelines for the use of prophylactic aspirin during pregnancy vary slightly among countries and groups. In this article, we review and summarize the evidence regarding the use of aspirin for PE prevention.
Figure
Reference
-
References
1. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010; 376:631–44.
Article2. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013; 122:1122–31.3. Irving RJ, Belton NR, Elton RA, Walker BR. Adult cardiovascular risk factors in premature babies. Lancet. 2000; 355:2135–6.
Article4. Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008; 359:262–73.
Article5. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017; 10:e003497.6. Breetveld NM, Ghossein-Doha C, van Neer J, Sengers MJJM, Geerts L, van Kuijk SMJ, et al. Decreased endothelial function and increased subclinical heart failure in women several years after pre-eclampsia. Ultrasound Obstet Gynecol. 2018; 52:196–204.
Article7. ACOG committee opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018; 132:e44–52.8. LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US preventive services task force recommendation statement. Ann Intern Med. 2014; 161:819–26.
Article9. Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007; 369:1791–8.
Article10. Staff AC, Benton SJ, von Dadelszen P, Roberts JM, Taylor RN, Powers RW, et al. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013; 61:932–42.
Article11. Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019; 15:275–89.
Article12. Herraiz I, Llurba E, Verlohren S, Galindo A. Spanish Group for the Study of Angiogenic Markers in Preeclampsia. Update on the diagnosis and prognosis of preeclampsia with the aid of the sFlt-1/ PlGF ratio in singleton pregnancies. Fetal Diagn Ther. 2018; 43:81–9.
Article13. Walsh SW. Eicosanoids in preeclampsia. Prostaglandins Leukot Essent Fatty Acids. 2004; 70:223–32.
Article14. Walsh SW. Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985; 152:335–40.
Article15. Moncada S, Vane JR. Arachidonic acid metabolites and the interactions between platelets and blood-vessel walls. N Engl J Med. 1979; 300:1142–7.
Article16. Mills JL, DerSimonian R, Raymond E, Morrow JD, Roberts LJ 2nd, Clemens JD, et al. Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: a multicenter prospective study. JAMA. 1999; 282:356–62.17. Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003; 110:255–8.
Article18. Bosetti F. Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models. J Neurochem. 2007; 102:577–86.
Article19. Patrono C. Aspirin and human platelets: from clinical trials to acetylation of cyclooxygenase and back. Trends Pharmacol Sci. 1989; 10:453–8.
Article20. Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol Suppl. 1997; 49:15–9.21. Fitzpatrick FA. Cyclooxygenase enzymes: regulation and function. Curr Pharm Des. 2004; 10:577–88.
Article22. Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998; 12:1063–73.
Article23. Clarke RJ, Mayo G, Price P, FitzGerald GA. Suppression of thromboxane A2 but not of systemic prostacyclin by controlled-release aspirin. N Engl J Med. 1991; 325:1137–41.
Article24. Lewis PJ, Boylan P, Friedman LA, Hensby CN, Downing I. Prostacyclin in pregnancy. Br Med J. 1980; 280:1581–2.
Article25. Fitzgerald DJ, Mayo G, Catella F, Entman SS, FitzGerald GA. Increased thromboxane biosynthesis in normal pregnancy is mainly derived from platelets. Am J Obstet Gynecol. 1987; 157:325–30.
Article26. Remuzzi G, Marchesi D, Zoja C, Muratore D, Mecca G, Misiani R, et al. Reduced umbilical and placental vascular prostacyclin in severe pre-eclampsia. Prostaglandins. 1980; 20:105–10.
Article27. Fitzgerald DJ, Entman SS, Mulloy K, FitzGerald GA. Decreased prostacyclin biosynthesis preceding the clinical manifestation of pregnancy-induced hypertension. Circulation. 1987; 75:956–63.
Article28. Craven LL. Prevention of coronary and cerebral thrombosis. Miss Valley Med J. 1956; 78:213–5.29. Goodlin RC, Haesslein HO, Fleming J. Aspirin for the treatment of recurrent toxaemia. Lancet. 1978; 2:51.30. Crandon AJ, Isherwood DM. Effect of aspirin on incidence of pre-eclampsia. Lancet. 1979; 1:1356.
Article31. Masotti G, Galanti G, Poggesi L, Abbate R, Neri Serneri GG. Differential inhibition of prostacyclin production and platelet aggregation by aspirin. Lancet. 1979; 2:1213–7.
Article32. Bussolino F, Benedetto C, Massobrio M, Camussi G. Maternal vascular prostacyclin activity in pre-eclampsia. Lancet. 1980; 2:702.
Article33. Beaufils M, Uzan S, Donsimoni R, Colau JC. Prevention of pre-eclampsia by early antiplatelet therapy. Lancet. 1985; 1:840–2.
Article34. Uzan S, Beaufils M, Breart G, Bazin B, Capitant C, Paris J. Prevention of fetal growth retardation with low-dose aspirin: findings of the EPREDA trial. Lancet. 1991; 337:1427–31.
Article35. CLASP Collaborative Group. CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet. 1994; 343:619–29.36. Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998; 338:701–5.
Article37. Duley L, Henderson-Smart D, Knight M, King J. Antiplatelet drugs for prevention of pre-eclampsia and its consequences: systematic review. BMJ. 2001; 322:329–33.
Article38. Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007; 369:1791–8.
Article39. Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010; 116:402–14.40. Meher S, Duley L, Hunter K, Askie L. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: an individual participant data meta-analysis. Am J Obstet Gynecol. 2017; 216:121–8e2.
Article41. Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017; 377:613–22.
Article42. Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol Int. 2013; 30:260–79.
Article43. World Health Organization (WHO). WHO recommendations or prevention and treatment of pre-eclampsia and eclampsia [Internet]. Geneva: WHO;c2011. [cited 2011 Nov 2 ]. Available from: https://www.who.int/publications/item/9789241548335 .44. Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013; 209:544e1–544.e12.
Article45. American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019; 133:e26–50.46. Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017; 216:110–20e6.
Article47. Chaemsaithong P, Cuenca-Gomez D, Plana MN, Gil MM, Poon LC. Does low-dose aspirin initiated before 11 weeks’ gestation reduce the rate of preeclampsia? Am J Obstet Gynecol. 2020; 222:437–50.
Article48. Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018; 218:287–93e1.
Article49. Francisco C, Wright D, Benkő Z, Syngelaki A, Nicolaides KH. Hidden high rate of pre-eclampsia in twin compared with singleton pregnancy. Ultrasound Obstet Gynecol. 2017; 50:88–92.
Article50. Bergeron TS, Roberge S, Carpentier C, Sibai B, McCaw-Binns A, Bujold E. Prevention of Preeclampsia with aspirin in multiple gestations: a systematic review and meta-analysis. Am J Perinatol. 2016; 33:605–10.
Article51. Euser AG, Metz TD, Allshouse AA, Heyborne KD. Low-dose aspirin for pre-eclampsia prevention in twins with elevated human chorionic gonadotropin. J Perinatol. 2016; 36:601–5.
Article52. Kalafat E, Shirazi A, Thilaganathan B, Khalil A. The role of aspirin in prevention of preeclampsia in twin pregnancies: does the dose matter? Am J Obstet Gynecol. 2020; 223:457–8.
Article53. Gent J, Nanda S, Khalil A, Sharp A. Antenatal management of multiple pregnancies within the UK: a survey of practice. Eur J Obstet Gynecol Reprod Biol. 2020; 254:74–8.
Article54. National Collaborating Centre for Women’s and Children’s Health (UK). Hypertension in pregnancy: the management of hypertensive disorders during pregnancy [Internet]. London: RCOG Press;c2010. [cited 2019 Nov 18]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK62652/ .55. Henderson JT, Vesco KK, Senger CA, Thomas RG, Redmond N. Aspirin use to prevent preeclampsia and related morbidity and mortality: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021; 326:1192–206.
Article56. O’Gorman N, Wright D, Poon LC, Rolnik DL, Syngelaki A, de Alvarado M, et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation: comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol. 2017; 49:756–60.
Article57. American College of Obstetricians and Gynecologists. Task force on hypertension in pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013; 122:1122–31.58. Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol. 2018; 51:743–50.
Article59. Poon LC, Nicolaides KH. Early prediction of preeclampsia. Obstet Gynecol Int. 2014; 2014:297397.
Article60. Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013; 33:8–15.
Article61. Chaemsaithong P, Sahota DS, Poon LC. First trimester preeclampsia screening and prediction. Am J Obstet Gynecol. 2022; 226:S1071–97e2.
Article62. O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am J Obstet Gynecol. 2016; 214:103e1–103.e12.
Article63. Rolnik DL, Wright D, Poon LCY, Syngelaki A, O’Gorman N, de Paco Matallana C, et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2017; 50:492–5.
Article64. Tan MY, Syngelaki A, Poon LC, Rolnik DL, O’Gorman N, Delgado JL, et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol. 2018; 52:186–95.
Article65. Agrawal S, Cerdeira AS, Redman C, Vatish M. Meta-analysis and systematic review to assess the role of soluble FMS-like tyrosine kinase-1 and placenta growth factor ratio in prediction of preeclampsia: the SaPPPhirE study. Hypertension. 2018; 71:306–16.
Article66. Cerdeira AS, Agrawal S, Staff AC, Redman CW, Vatish M. Angiogenic factors: potential to change clinical practice in pre-eclampsia? BJOG. 2018; 125:1389–95.
Article67. Bian X, Biswas A, Huang X, Lee KJ, Li TK, Masuyama H, et al. Short-term prediction of adverse outcomes using the sFlt-1 (soluble fms-like tyrosine kinase 1)/PlGF (placental growth factor) ratio in Asian women with suspected preeclampsia. Hypertension. 2019; 74:164–72.
Article68. Gallo DM, Wright D, Casanova C, Campanero M, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 19–24 weeks’ gestation. Am J Obstet Gynecol. 2016; 214:619e1–17.
Article69. Litwinska M, Syngelaki A, Wright A, Wright D, Nicolaides KH. Management of pregnancies after combined screening for pre-eclampsia at 19–24 weeks’ gestation. Ultrasound Obstet Gynecol. 2018; 52:365–72.70. Salihu HM, Garcia BY, Dongarwar D, Maiyegun SO, Yusuf KK, Agili DEA. Maternal pre-pregnancy underweight and the risk of small-for-gestational-age in Asian-American ethnic groups. Obstet Gynecol Sci. 2021; 64:496–505.
Article71. Wadhwani P, Saha PK, Kalra JK, Gainder S, Sundaram V. A study to compare maternal and perinatal outcome in early vs. late onset preeclampsia. Obstet Gynecol Sci. 2020; 63:270–7.
Article72. Ebrashy A, Ibrahim M, Marzook A, Yousef D. Usefulness of aspirin therapy in high-risk pregnant women with abnormal uterine artery Doppler ultrasound at 14–16 weeks pregnancy: randomized controlled clinical trial. Croat Med J. 2005; 46:826–31.73. Goffinet F, Bréart G, Uzan S. ECPPA: randomised trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnant women. Br J Obstet Gynaecol. 1996; 103:719–20.
Article74. Golding J. Jamaica Low Dose Aspirin Study Group. A randomised trial of low dose aspirin for primiparae in pregnancy. Br J Obstet Gynaecol. 1998; 105:293–9.
Article75. Hermida RC, Ayala DE, Fernández JR, Mojón A, Alonso I, Silva I, et al. Administration time-dependent effects of aspirin in women at differing risk for preeclampsia. Hypertension. 1999; 34:1016–23.
Article76. Michael CA, Walters BNJ. Low-dose aspirin in the prevention of pre-eclampsia: current evaluation. Maternal Physiology and Pathology. 1992; 183–9.77. Morris JM, Fay RA, Ellwood DA, Cook CM, Devonald KJ. A randomized controlled trial of aspirin in patients with abnormal uterine artery blood flow. Obstet Gynecol. 1996; 87:74–8.
Article78. Villa PM, Kajantie E, Räikkönen K, Pesonen AK, Hämäläinen E, Vainio M, et al. Aspirin in the prevention of pre-eclampsia in high-risk women: a randomised placebo-controlled PREDO trial and a meta-analysis of randomised trials. BJOG. 2013; 120:64–74.
Article79. Zhao YM, Xiao LP, Hu H, Yang XN, Xu YQ, Guo LM. Low-dose aspirin prescribed at bed time for the prevention of pre-eclampsia in high-risk pregnant women. Reprod Contracept. 2012; 32:355–9.80. Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Canadian Hypertensive Disorders of Pregnancy (HDP) Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014; 4:105–45.
Article81. Lowe SA, Bowyer L, Lust K, McMahon LP, Morton MR, North RA, et al. The SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol. 2015; 55:11–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Treatment with Low-dose Aspirin in the Mild Preeclamptic Patients in the Third Trimester of Pregnancy
- Thromboxane in Pregnancy-Induced Hypertension
- A Comparative Study of Therapeutic Effect of Intravenous Gammaglobulin plus Aspirin Versus Aspirin Alone in Kawasaki Syndrome
- Anesthetic management for preeclampsia: Hemodynamic monitoring and volume therapy
- Aspirin Resistance in Patients Undergoing Dialysis