J Korean Med Sci.
2023 May;38(17):e135. 10.3346/jkms.2023.38.e135.
Injectable Poloxamer Hydrogel Formulations for Intratympanic Delivery of Dexamethasone
- Affiliations
-
- 1Department of Polymer Science and Engineering, Chungnam National University, Daejeon, Korea
- 2Department of Otolaryngology-Head and Neck Surgery, College of Medicine, Chungnam National University, Daejeon, Korea
- 3Department of Medical Science, College of Medicine, Chungnam National University, Daejeon, Korea
- 4Brain Research Institute, College of Medicine, Chungnam National University, Daejeon, Korea
- KMID: 2542023
- DOI: http://doi.org/10.3346/jkms.2023.38.e135
Abstract
- Background
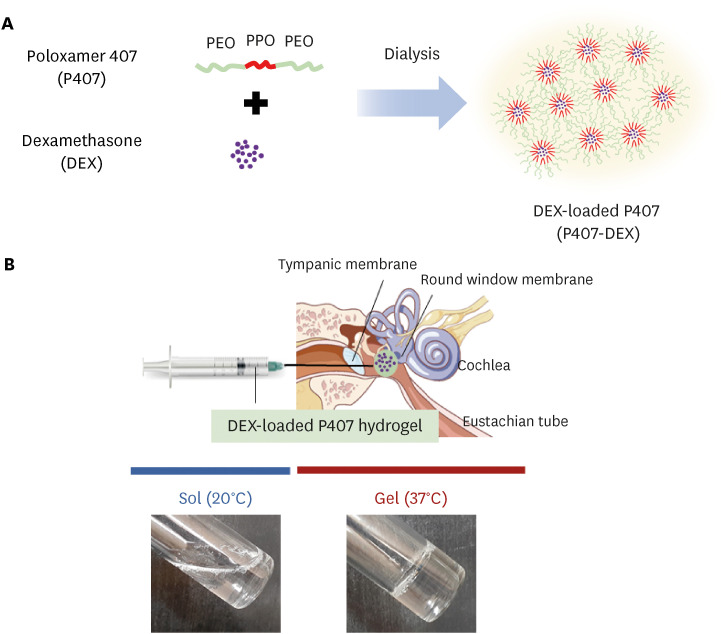
In this study, we prepared and evaluated an injectable poloxamer (P407) hydrogel formulation for intratympanic (IT) delivery of dexamethasone (DEX).
Methods
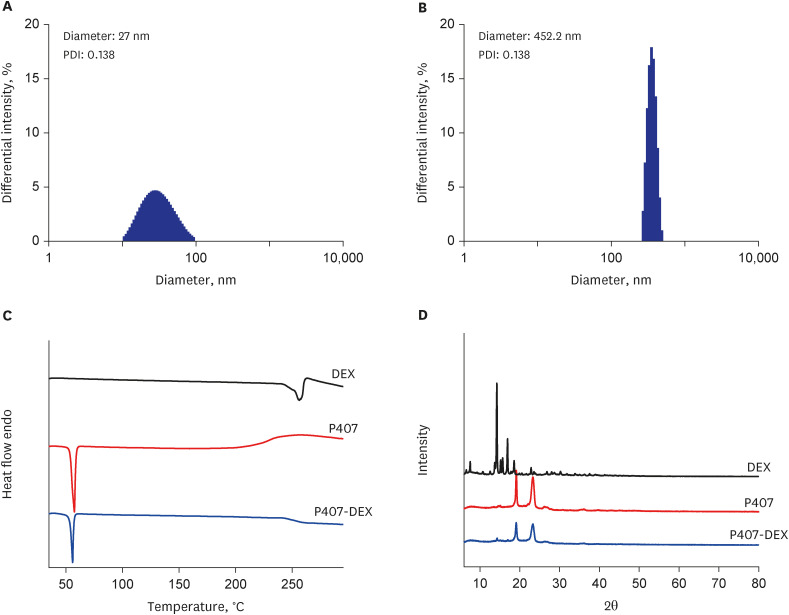
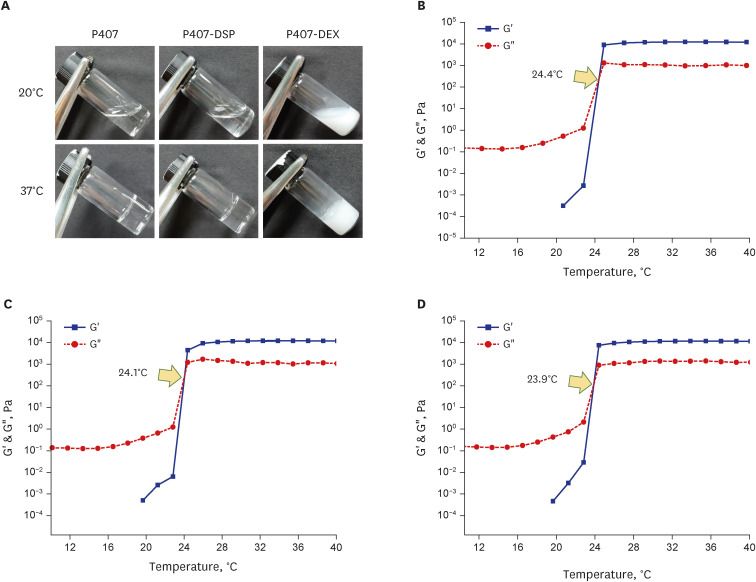
DEX-loaded P407 hydrogels were characterized in terms of thermogelation, drug loading capacities, particle size, and drug release. The in vivo toxicity and drug absorption of the DEX-loaded P407 formulation after IT injection were evaluated using an animal model by performing histopathological analysis and drug concentration measurements.
Results
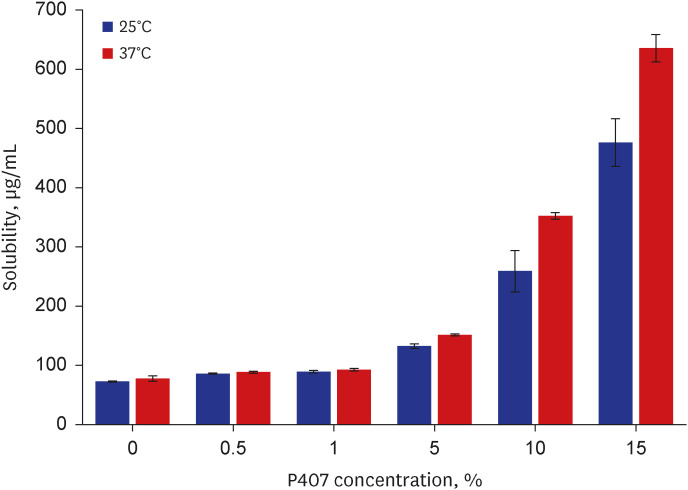
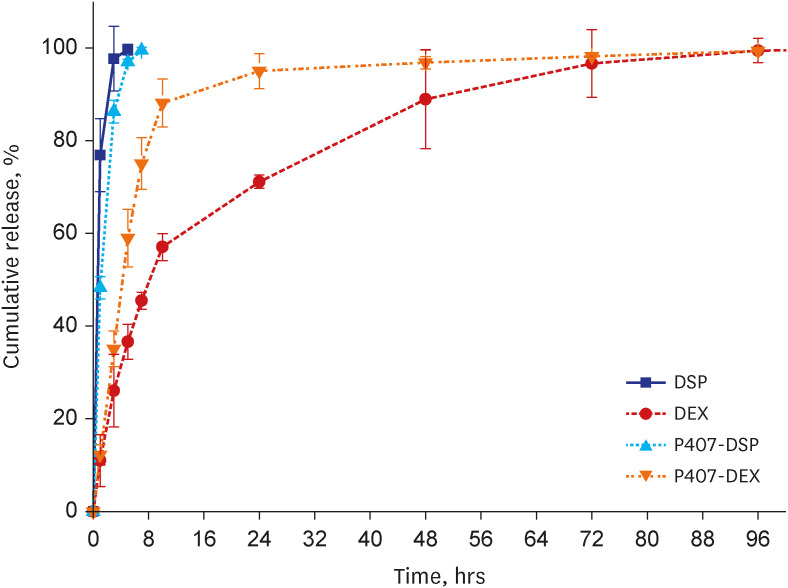
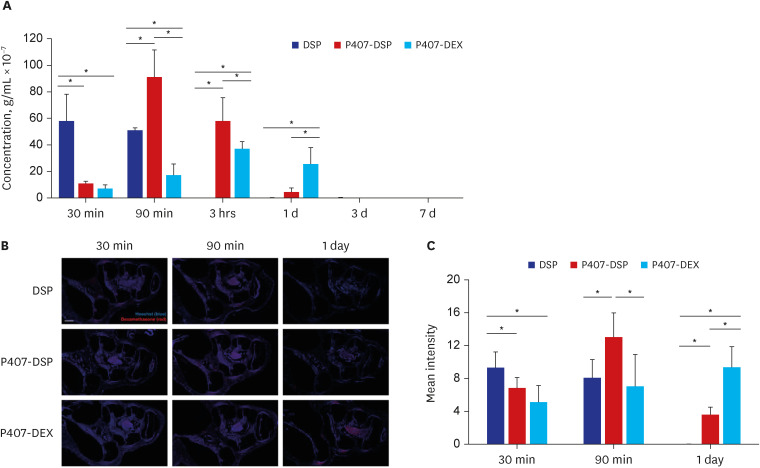
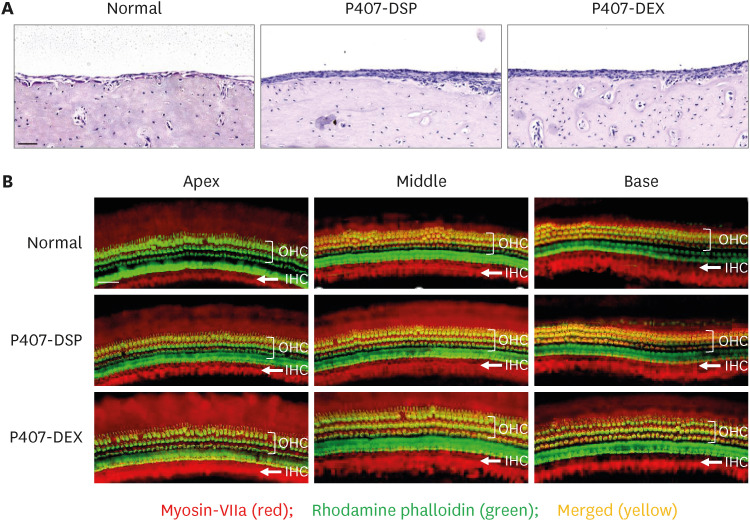
The P407 hydrogel effectively solubilized hydrophobic DEX and demonstrated a sustained release compared to the hydrophilic DEX formulation. The in vivo study showed that the hydrogel formulation delivered considerable drug concentrations to the inner ear and displayed a favorable safety profile without apparent cytotoxicity or inflammation.
Conclusion
P407 hydrogel can be useful as an injectable inner ear delivery formulation for hydrophobic drugs due to their biocompatibility, drug-solubilizing capacity, thermogelation, and controlled release.
Figure
Reference
-
1. Liu H, Hao J, Li KS. Current strategies for drug delivery to the inner ear. Acta Pharm Sin B. 2013; 3(2):86–96.
Article2. Saito T, Zhang ZJ, Tokuriki M, Ohtsubo T, Noda I, Shibamori Y, et al. Expression of p-glycoprotein is associated with that of multidrug resistance protein 1 (MRP1) in the vestibular labyrinth and endolymphatic sac of the guinea pig. Neurosci Lett. 2001; 303(3):189–192. PMID: 11323117.
Article3. McCall AA, Swan EE, Borenstein JT, Sewell WF, Kujawa SG, McKenna MJ. Drug delivery for treatment of inner ear disease: current state of knowledge. Ear Hear. 2010; 31(2):156–165. PMID: 19952751.
Article4. Pararas EE, Borkholder DA, Borenstein JT. Microsystems technologies for drug delivery to the inner ear. Adv Drug Deliv Rev. 2012; 64(14):1650–1660. PMID: 22386561.
Article5. Honeder C, Engleder E, Schöpper H, Gabor F, Reznicek G, Wagenblast J, et al. Sustained release of triamcinolone acetonide from an intratympanically applied hydrogel designed for the delivery of high glucocorticoid doses. Audiol Neurootol. 2014; 19(3):193–202. PMID: 24714604.
Article6. Li L, Chao T, Brant J, O’Malley B Jr, Tsourkas A, Li D. Advances in nano-based inner ear delivery systems for the treatment of sensorineural hearing loss. Adv Drug Deliv Rev. 2017; 108:2–12. PMID: 26796230.
Article7. Ferreira NN, Ferreira LM, Cardoso VM, Boni F, Souza AL, Gremião MP. Recent advances in smart hydrogels for biomedical applications: from self-assembly to functional approaches. Eur Polym J. 2018; 99:117–133.
Article8. Wei M, Gao Y, Li X, Serpe MJ. Stimuli-responsive polymers and their applications. Polym Chem. 2017; 8(1):127–143.
Article9. Qureshi D, Nayak SK, Maji S, Anis A, Kim D, Pal K. Environment sensitive hydrogels for drug delivery applications. Eur Polym J. 2019; 120:109220.
Article10. Rathnam C, Chueng SD, Ying YM, Lee KB, Kwan K. Developments in bio-inspired nanomaterials for therapeutic delivery to treat hearing loss. Front Cell Neurosci. 2019; 13:493. PMID: 31780898.
Article11. Cho IS, Park CG, Huh BK, Cho MO, Khatun Z, Li Z, et al. Thermosensitive hexanoyl glycol chitosan-based ocular delivery system for glaucoma therapy. Acta Biomater. 2016; 39:124–132. PMID: 27163401.
Article12. Patel P, Mandal A, Gote V, Pal D, Mitra AK. Thermosensitive hydrogel-based drug delivery system for sustained drug release. J Polym Res. 2019; 26(6):1–11.
Article13. Xiao Y, Gu Y, Qin L, Chen L, Chen X, Cui W, et al. Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf B Biointerfaces. 2021; 200:111581. PMID: 33524696.
Article14. Paulson DP, Abuzeid W, Jiang H, Oe T, O’Malley BW, Li D. A novel controlled local drug delivery system for inner ear disease. Laryngoscope. 2008; 118(4):706–711. PMID: 18182968.
Article15. Saravanan S, Vimalraj S, Thanikaivelan P, Banudevi S, Manivasagam G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int J Biol Macromol. 2019; 121:38–54. PMID: 30291931.
Article16. Bodratti AM, Alexandridis P. Formulation of poloxamers for drug delivery. J Funct Biomater. 2018; 9(1):11. PMID: 29346330.
Article17. Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006; 23(12):2709–2728. PMID: 17096184.
Article18. Piu F, Bishop KM. Local drug delivery for the treatment of neurotology disorders. Front Cell Neurosci. 2019; 13:238. PMID: 31213983.
Article19. Ricci EJ, Lunardi LO, Nanclares DM, Marchetti JM. Sustained release of lidocaine from Poloxamer 407 gels. Int J Pharm. 2005; 288(2):235–244. PMID: 15620863.
Article20. Dumortier G, El Kateb N, Sahli M, Kedjar S, Boulliat A, Chaumeil JC. Development of a thermogelling ophthalmic formulation of cysteine. Drug Dev Ind Pharm. 2006; 32(1):63–72. PMID: 16455605.
Article21. Silverstein H, Choo D, Rosenberg SI, Kuhn J, Seidman M, Stein I. Intratympanic steroid treatment of inner ear disease and tinnitus (preliminary report). Ear Nose Throat J. 1996; 75(8):468–471. PMID: 8828271.
Article22. Gao Z, Schwieger J, Matin-Mann F, Behrens P, Lenarz T, Scheper V. Dexamethasone for inner ear therapy: biocompatibility and bio-efficacy of different dexamethasone formulations in vitro. Biomolecules. 2021; 11(12):1896. PMID: 34944539.
Article23. Xu K, Chen S, Xie L, Qiu Y, Liu XZ, Bai X, et al. The protective effects of systemic dexamethasone on sensory epithelial damage and hearing loss in targeted Cx26-null mice. Cell Death Dis. 2022; 13(6):545. PMID: 35688810.
Article24. Huh KM, Lee SC, Cho YW, Lee J, Jeong JH, Park K. Hydrotropic polymer micelle system for delivery of paclitaxel. J Control Release. 2005; 101(1-3):59–68. PMID: 15588894.
Article25. Schmolka IR. Artificial skin. I. Preparation and properties of pluronic F-127 gels for treatment of burns. J Biomed Mater Res. 1972; 6(6):571–582. PMID: 4642986.
Article26. Yu Y, Kim DH, Suh EY, Jeong SH, Kwon HC, Le TP, et al. Injectable glycol chitosan thermogel formulation for efficient inner ear drug delivery. Carbohydr Polym. 2022; 278:118969. PMID: 34973784.
Article27. Lyu AR, Kim DH, Lee SH, Shin DS, Shin SA, Park YH. Effects of dexamethasone on intracochlear inflammation and residual hearing after cochleostomy: a comparison of administration routes. PLoS One. 2018; 13(3):e0195230. PMID: 29601595.
Article28. Kolašinac N, Kachrimanis K, Homšek I, Grujić B, Ðurić Z, Ibrić S. Solubility enhancement of desloratadine by solid dispersion in poloxamers. Int J Pharm. 2012; 436(1-2):161–170. PMID: 22772487.
Article29. Zarrintaj P, Ramsey JD, Samadi A, Atoufi Z, Yazdi MK, Ganjali MR, et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020; 110:37–67. PMID: 32417265.
Article30. Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope. 1999; 109(7 Pt 2):1–17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Development of Injectable Intraocular Lens Using Thermosensitive Polymeric Hydrogel in Vitro and in Vivo
- IrreVersible Gel Tansformation of Injectavble Intraocular Lens by Photoinitiator and UV Irradiation in Rabbits
- Poloxamer 407 Hydrogels for Intravesical Instillation to Mouse Bladder: Gel-Forming Capacity and Retention Performance
- The Comparison for the Growth of Microorganisms in Original Propofol, Ethylenediaminetetraacetic Acid (EDTA) Added Propofol, and Poloxamer-Solutol Formulated Propofol
- Injectable and Cell-Laden Hydrogel in the Contained Bone Defect Animal Model: A Systematic Review