J Korean Med Sci.
2023 Apr;38(14):e106. 10.3346/jkms.2023.38.e106.
COVID-19 Vaccine-Associated Pneumonitis in the Republic of Korea: A Nationwide Multicenter Survey
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 3Division of Allergy, Pulmonary and Critical Care Medicine, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- 4Division of Allergy and Respiratory Medicine, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea
- 5Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, School of Medicine, Inha University, Incheon, Korea
- 6Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Busan Paik Hospital, Inje University, Busan, Korea
- 7Division of Pulmonary, Department of Internal Medicine, College of Medicine, Wonkwang University, Iksan, Korea
- 8Division of Pulmonary and Critical Medicine, Department of Internal Medicine, Ulsan University Hospital, College of Medicine, University of Ulsan, Ulsan, Korea
- 9Department of Internal Medicine, Gyeongsang National University Hospital, Jinju, Korea
- 10Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 11Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
- 12Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, College of Medicine, Seoul National University, Seoul, Korea
- 13Division of Respiratory-Allergy and Clinical Immunology, Department of Internal Medicine, Konkuk University Medical Center, Seoul, Korea
- 14Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 15Department of Pulmonary and Critical Care Medicine, Ajou University School of Medicine, Suwon, Korea
- 16Department of Internal Medicine, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea
- 17Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Kyunghee University Hospital, Seoul, Korea
- KMID: 2541548
- DOI: http://doi.org/10.3346/jkms.2023.38.e106
Abstract
- Background
Recent reports have suggested that pneumonitis is a rare complication following vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, its clinical features and outcomes are not well known. The aim of this study was to identify the clinical characteristics and outcomes of patients with vaccine-associated pneumonitis following vaccination against SARS-CoV-2.
Methods
In this nationwide multicenter survey study, questionnaires were distributed to pulmonary physicians in referral hospitals. They were asked to report cases of development or exacerbation of interstitial lung disease (ILD) associated with the coronavirus disease 2019 vaccine. Vaccine-associated pneumonitis was defined as new pulmonary infiltrates documented on chest computed tomography within 4 weeks of vaccination and exclusion of other possible etiologies.
Results
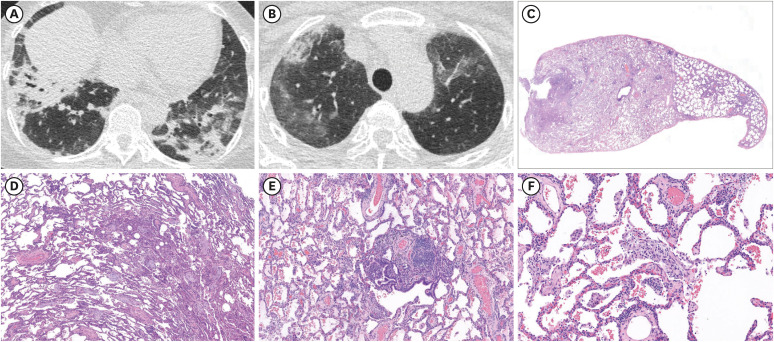
From the survey, 49 cases of vaccine-associated pneumonitis were identified between February 27 and October 30, 2021. After multidisciplinary discussion, 46 cases were analyzed. The median age was 66 years and 28 (61%) were male. The median interval between vaccination and respiratory symptoms was 5 days. There were 20 (43%), 17 (37%), and nine (19%) patients with newly identified pneumonitis, exacerbation of pre-diagnosed ILD, and undetermined pre-existing ILD, respectively. The administered vaccines were BNT162b2 and ChAdOx1 nCov-19/AZD1222 each in 21 patients followed by mRNA-1273 in three, and Ad26. COV2.S in one patient. Except for five patients with mild disease, 41 (89%) patients were treated with corticosteroid. Significant improvement was observed in 26 (57%) patients including four patients who did not receive treatment. However, ILD aggravated in 9 (20%) patients despite treatment. Mortality was observed in eight (17%) patients.
Conclusion
These results suggest pneumonitis as a potentially significant safety concern for vaccines against SARS-CoV-2. Clinical awareness and patient education are necessary for early recognition and prompt management. Additional research is warranted to identify the epidemiology and characterize the pathophysiology of vaccine-associated pneumonitis.
Keyword
Figure
Reference
-
1. World Health Organization. Weekly epidemiological update on COVID-19 - 6. July, 2022. Updated 2022. Accessed July 9, 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---6-july-2022 .2. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021; 21(10):626–636. PMID: 34373623.
Article3. Wu Q, Dudley MZ, Chen X, Bai X, Dong K, Zhuang T, et al. Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC Med. 2021; 19(1):173. PMID: 34315454.4. Yan ZP, Yang M, Lai CL. COVID-19 vaccines: a review of the safety and efficacy of current clinical trials. Pharmaceuticals (Basel). 2021; 14(5):406. PMID: 33923054.
Article5. Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021; 384(23):2202–2211. PMID: 33861525.
Article6. Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(27):977–982. PMID: 34237049.7. Woo EJ, Mba-Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of receipt of the Ad26.COV2.S COVID-19 vaccine with presumptive Guillain-Barré syndrome, February-July 2021. JAMA. 2021; 326(16):1606–1613. PMID: 34617967.8. Park JY, Kim JH, Lee IJ, Kim HI, Park S, Hwang YI, et al. COVID-19 vaccine-related interstitial lung disease: a case study. Thorax. 2022; 77(1):102–104. PMID: 34362838.9. Yoshifuji A, Ishioka K, Masuzawa Y, Suda S, Murata S, Uwamino Y, et al. COVID-19 vaccine induced interstitial lung disease. J Infect Chemother. 2022; 28(1):95–98. PMID: 34580010.
Article10. Matsuzaki S, Kamiya H, Inoshima I, Hirasawa Y, Tago O, Arai M. COVID-19 mRNA vaccine-induced pneumonitis. Intern Med. 2022; 61(1):81–86. PMID: 34707048.
Article11. Kono A, Yoshioka R, Hawk P, Iwashina K, Inoue D, Suzuki M, et al. A case of severe interstitial lung disease after COVID-19 vaccination. QJM. 2022; 114(11):805–806. PMID: 34618126.
Article12. Stoyanov A, Thompson G, Lee M, Katelaris C. Delayed hypersensitivity to the Comirnaty coronavirus disease 2019 vaccine presenting with pneumonitis and rash. Ann Allergy Asthma Immunol. 2022; 128(3):321–322. PMID: 34813953.
Article13. Miqdadi A, Herrag M. Acute eosinophilic pneumonia associated with the anti-COVID-19 vaccine AZD1222. Cureus. 2021; 13(10):e18959. PMID: 34812326.
Article14. Ueno T, Ohta T, Sugio Y, Ohno Y, Uehara Y. Severe acute interstitial lung disease after BNT162b2 mRNA COVID-19 vaccination in a patient post HLA-haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant. 2022; 57(5):840–842. PMID: 35273388.
Article15. Barrio Piqueras M, Ezponda A, Felgueroso C, Urtasun C, Di Frisco IM, Larrache JC, et al. Acute eosinophilic pneumonia following mRNA COVID-19 vaccination: a case report. Arch Bronconeumol. 2022; 58:53–54. PMID: 34803207.16. Shimizu T, Watanabe S, Yoneda T, Kinoshita M, Terada N, Kobayashi T, et al. Interstitial pneumonitis after COVID-19 vaccination: a report of three cases. Allergol Int. 2022; 71(2):251–253. PMID: 34772608.17. Yoshimura Y, Sasaki H, Miyata N, Miyazaki K, Okudela K, Tateishi Y, et al. An autopsy case of COVID-19-like acute respiratory distress syndrome after mRNA-1273 SARS-CoV-2 vaccination. Int J Infect Dis. 2022; 121:98–101. PMID: 35500794.
Article18. Sgalla G, Magrì T, Lerede M, Comes A, Richeldi L. COVID-19 vaccine in patients with exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2022; 206(2):219–221. PMID: 35412453.
Article19. Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. 2012; 13(1):39. PMID: 22651223.
Article20. Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. 2018; 7(10):356. PMID: 30326612.
Article21. Akoun GM, Cadranel JL, Rosenow EC 3rd, Milleron BJ. Bronchoalveolar lavage cell data in drug-induced pneumonitis. Allerg Immunol (Paris). 1991; 23(6):245–252. PMID: 1878140.22. Rocco A, Sgamato C, Compare D, Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casuality. J Hepatol. 2021; 75(3):728–729. PMID: 34116081.
Article23. Li X, Tong X, Yeung WW, Kuan P, Yum SH, Chui CS, et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2022; 81(4):564–568. PMID: 34686479.
Article24. Khan S, Shafiei MS, Longoria C, Schoggins JW, Savani RC, Zaki H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. eLife. 2021; 10:e68563. PMID: 34866574.25. Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021; 21(8):475–484. PMID: 34211186.26. Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022; 114:252–260. PMID: 34800687.27. Lee H, Choi H, Yang B, Lee SK, Park TS, Park DW, et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur Respir J. 2021; 58(6):2004125. PMID: 33888524.28. Watanabe S, Waseda Y, Takato H, Inuzuka K, Katayama N, Kasahara K, et al. Influenza vaccine-induced interstitial lung disease. Eur Respir J. 2013; 41(2):474–477. PMID: 23370803.29. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015; 33(36):4398–4405. PMID: 26209838.30. Kochhar S, Excler JL, Bok K, Gurwith M, McNeil MM, Seligman SJ, et al. Defining the interval for monitoring potential adverse events following immunization (AEFIs) after receipt of live viral vectored vaccines. Vaccine. 2019; 37(38):5796–5802. PMID: 30497831.31. Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021; 385(18):1680–1689. PMID: 34379914.
Article32. Müller NL, White DA, Jiang H, Gemma A. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer. 2004; 91(Suppl 2):S24–S30. PMID: 15340375.
Article33. Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am J Respir Crit Care Med. 2016; 194(3):265–275. PMID: 27299520.34. Zhu S, Fu Y, Zhu B, Zhang B, Wang J. Pneumonitis induced by immune checkpoint inhibitors: from clinical data to translational investigation. Front Oncol. 2020; 10:1785. PMID: 33042827.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Delphi Survey for COVID-19 Vaccination in Korean Children Between 5 and 11 Years Old
- Therapeutics in the Treatment of COVID-19 for Children and Adolescents
- Association of COVID-19 vaccine attitudes and cognitions of COVID-19-related stigma with vaccine hesitancy among college students
- COVID-19 Vaccination for Pilots and Air Traffic Controllers
- Updates on Coronavirus Disease 19 Vaccine and Its Clinical Application