Cancer Res Treat.
2023 Apr;55(2):429-441. 10.4143/crt.2022.891.
Validation and Clinical Application of ONCOaccuPanel for Targeted Next-Generation Sequencing of Solid Tumors
- Affiliations
-
- 1Department of Pathology, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 2Bioinformatics Team, R&D Center, NGeneBio Inc., Seoul, Korea
- 3Diagnostics Development A Team, R&D Center, NGeneBio Inc., Seoul, Korea
- KMID: 2541230
- DOI: http://doi.org/10.4143/crt.2022.891
Abstract
- Purpose
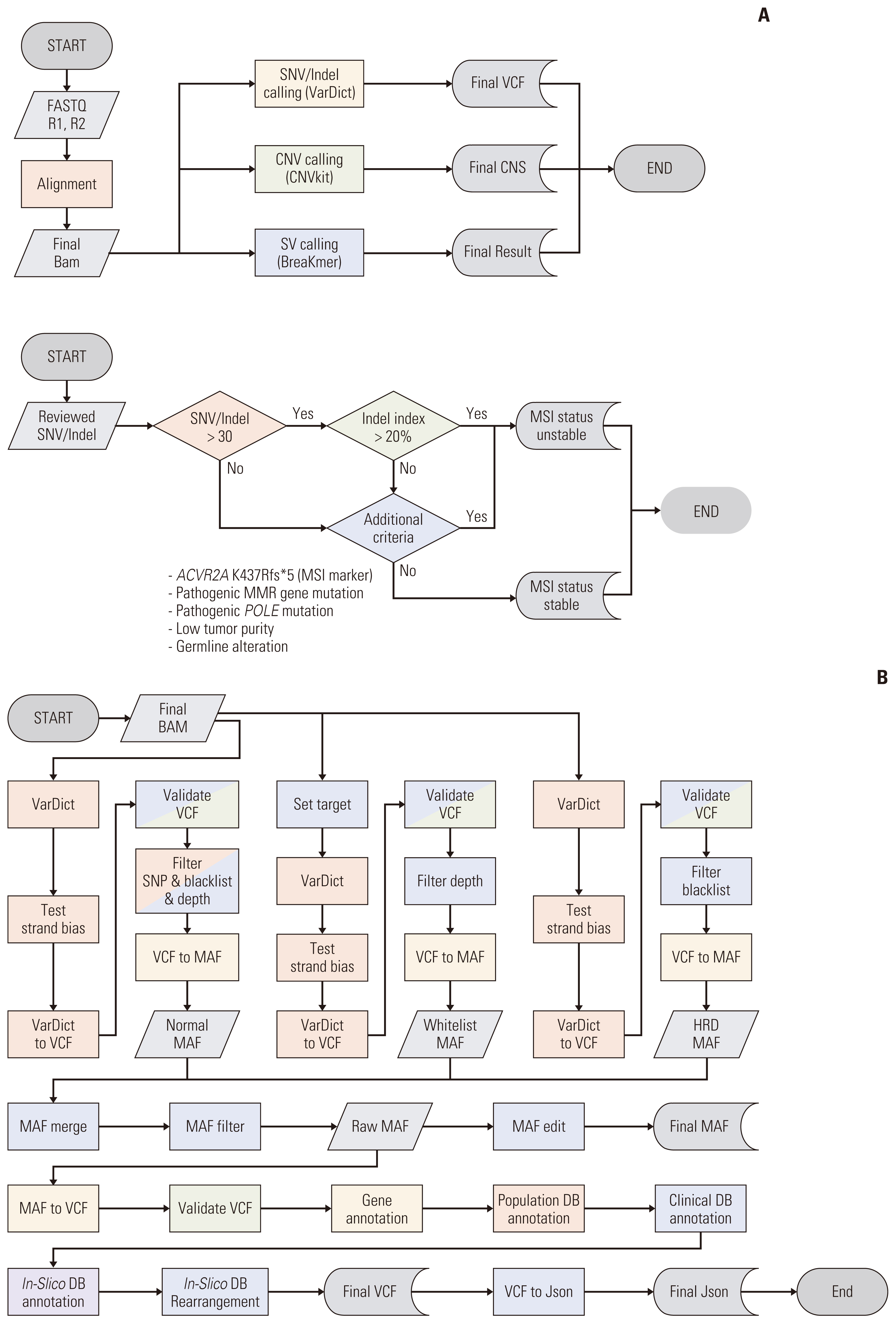
Targeted next-generation sequencing (NGS) is widely used for simultaneously detecting clinically informative genetic alterations in a single assay. Its application in clinical settings requires the validation of NGS gene panels. In this study, we aimed to validate a targeted hybridization capture-based DNA panel (ONCOaccuPanel) using the Illumina MiSeq sequencing platform. The panel allows the simultaneous detection of single-nucleotide variants (SNVs), insertions, deletions, and copy number changes of 323 genes and fusions of 17 genes in solid tumors.
Materials and Methods
We used 16 formalin-fixed paraffin-embedded (FFPE) tumor samples with previously known genetic mutations and one reference material (HD827) for validation. Moreover, we sequenced an additional 117 FFPE tumor samples to demonstrate the clinical utility of this panel.
Results
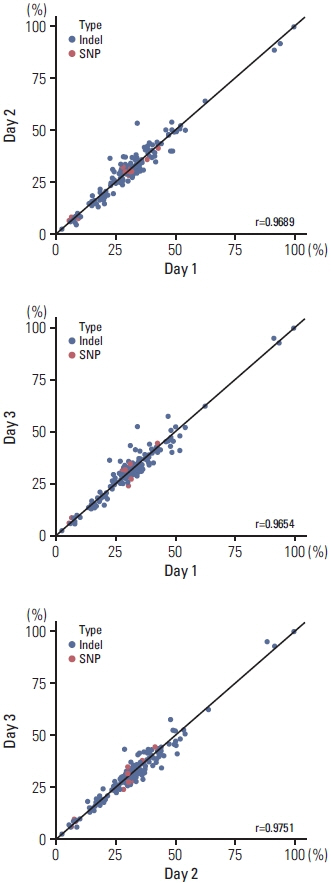
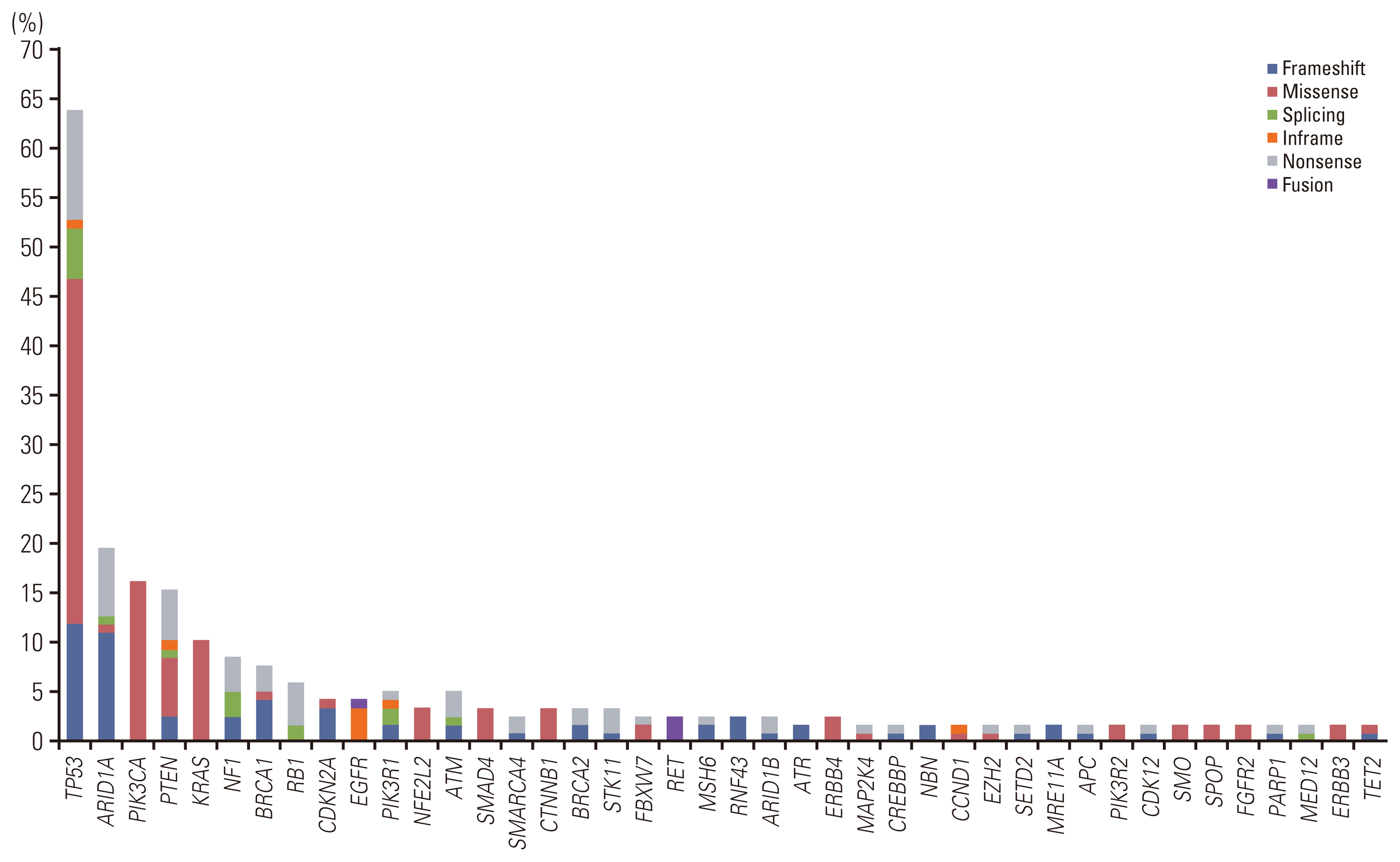
Validation revealed a 100% positive percentage agreement and positive predictive value for the detection of SNVs, insertions, deletions, copy number changes, fusion genes, and microsatellite instability–high types. We observed high levels of reproducibility and repeatability (R2 correlation coefficients=0.96-0.98). In the limit of detection assessment, we identified all clinically relevant genes with allele frequencies > 3%. Furthermore, the clinical application of ONCOaccuPanel using 117 FFPE samples demonstrated robust detection of oncogenic alterations. Oncogenic alterations and targetable genetic alterations were detected in 98.2% and 27.4% cases, respectively.
Conclusion
ONCOaccuPanel demonstrated high analytical sensitivity, reproducibility, and repeatability and is feasible for the detection of clinically relevant mutations in clinical settings.
Keyword
Figure
Cited by 1 articles
-
BRCA -mutated gastric adenocarcinomas are associated with chromosomal instability and responsiveness to platinum-based chemotherapy
Ji Hyun Oh, Chang Ohk Sung, Hyung-Don Kim, Sung-Min Chun, Jihun Kim
J Pathol Transl Med. 2023;57(6):323-331. doi: 10.4132/jptm.2023.10.22.
Reference
-
References
1. Chakravarty D, Solit DB. Clinical cancer genomic profiling. Nat Rev Genet. 2021; 22:483–501.
Article2. Yatabe Y, Sunami K, Goto K, Nishio K, Aragane N, Ikeda S, et al. Multiplex gene-panel testing for lung cancer patients. Pathol Int. 2020; 70:921–31.
Article3. Pennell NA, Mutebi A, Zhou ZY, Ricculli ML, Tang W, Wang H, et al. Economic impact of next-generation sequencing versus single-gene testing to detect genomic alterations in metastatic non-small-cell lung cancer using a decision analytic model. JCO Precis Oncol. 2019; 3:1–9.
Article4. Heist RS, Shim HS, Gingipally S, Mino-Kenudson M, Le L, Gainor JF, et al. MET exon 14 skipping in non-small cell lung cancer. Oncologist. 2016; 21:481–6.5. Kohno T, Nakaoku T, Tsuta K, Tsuchihara K, Matsumoto S, Yoh K, et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015; 4:156–64.6. Vaishnavi A, Capelletti M, Le AT, Kako S, Butaney M, Ercan D, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013; 19:1469–72.
Article7. Skoulidis F, Heymach JV. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer. 2019; 19:495–509.
Article8. Polyak K. Heterogeneity in breast cancer. J Clin Invest. 2011; 121:3786–8.
Article9. Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet. 2016; 17:333–51.
Article10. Jennings LJ, Arcila ME, Corless C, Kamel-Reid S, Lubin IM, Pfeifer J, et al. Guidelines for validation of next-generation sequencing-based oncology panels: a joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn. 2017; 19:341–65.11. Aziz N, Zhao Q, Bry L, Driscoll DK, Funke B, Gibson JS, et al. College of American Pathologists’ laboratory standards for next-generation sequencing clinical tests. Arch Pathol Lab Med. 2015; 139:481–93.
Article12. Rehm HL, Bale SJ, Bayrak-Toydemir P, Berg JS, Brown KK, Deignan JL, et al. ACMG clinical laboratory standards for next-generation sequencing. Genet Med. 2013; 15:733–47.
Article13. Gargis AS, Kalman L, Berry MW, Bick DP, Dimmock DP, Hambuch T, et al. Assuring the quality of next-generation sequencing in clinical laboratory practice. Nat Biotechnol. 2012; 30:1033–6.
Article14. Kim J, Park WY, Kim NK, Jang SJ, Chun SM, Sung CO, et al. Good laboratory standards for clinical next-generation sequencing cancer panel tests. J Pathol Transl Med. 2017; 51:191–204.
Article15. Roy S, Coldren C, Karunamurthy A, Kip NS, Klee EW, Lincoln SE, et al. Standards and guidelines for validating next-generation sequencing bioinformatics pipelines: a joint recommendation of the Association for Molecular Pathology and the College of American Pathologists. J Mol Diagn. 2018; 20:4–27.
Article16. Kim JE, Chun SM, Hong YS, Kim KP, Kim SY, Kim J, et al. Mutation burden and I index for detection of microsatellite instability in colorectal cancer by targeted next-generation sequencing. J Mol Diagn. 2019; 21:241–50.
Article17. Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004; 96:261–8.
Article18. Luchini C, Bibeau F, Ligtenberg MJ, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019; 30:1232–43.
Article19. Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017; 19:4–23.
Article20. Nakamura Y, Kawazoe A, Lordick F, Janjigian YY, Shitara K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol. 2021; 18:473–87.
Article21. Denny JC, Collins FS. Precision medicine in 2030-seven ways to transform healthcare. Cell. 2021; 184:1415–9.
Article22. Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet. 2010; 11:685–96.
Article23. Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015; 17:251–64.24. Bolivar AM, Luthra R, Mehrotra M, Chen W, Barkoh BA, Hu P, et al. Targeted next-generation sequencing of endometrial cancer and matched circulating tumor DNA: identification of plasma-based, tumor-associated mutations in early stage patients. Mod Pathol. 2019; 32:405–14.
Article25. Cancer Genome Atlas Research Network; Albert Einstein College of Medicine; Analytical Biological Services; Barretos Cancer Hospital; Baylor College of Medicine; Beckman Research Institute of City of Hope, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017; 543:378–84.26. Vang R, Levine DA, Soslow RA, Zaloudek C, Shih Ie M, Kurman RJ. Molecular alterations of TP53 are a defining feature of ovarian high-grade serous carcinoma: a rereview of cases lacking TP53 mutations in the Cancer Genome Atlas Ovarian Study. Int J Gynecol Pathol. 2016; 35:48–55.
Article27. Ross JS, Ali SM, Wang K, Palmer G, Yelensky R, Lipson D, et al. Comprehensive genomic profiling of epithelial ovarian cancer by next generation sequencing-based diagnostic assay reveals new routes to targeted therapies. Gynecol Oncol. 2013; 130:554–9.
Article28. Wei S, Hu M, Yang Y, Huang X, Li B, Ding L, et al. Case report: short-term response to first-line crizotinib monotherapy in a metastatic lung adenocarcinoma patient harboring a novel TPR-ROS1 fusion. Front Oncol. 2022; 12:862008.
Article29. Kao YC, Suurmeijer AJ, Argani P, Dickson BC, Zhang L, Sung YS, et al. Soft tissue tumors characterized by a wide spectrum of kinase fusions share a lipofibromatosis-like neural tumor pattern. Genes Chromosomes Cancer. 2020; 59:575–83.
Article30. Pascual J, Turner NC. Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann Oncol. 2019; 30:1051–60.
Article31. Hastings PJ, Lupski JR, Rosenberg SM, Ira G. Mechanisms of change in gene copy number. Nat Rev Genet. 2009; 10:551–64.
Article32. Pilie PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019; 16:81–104.
Article33. Li K, Luo H, Huang L, Luo H, Zhu X. Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int. 2020; 20:16.
Article34. Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006; 66:7810–7.
Article35. Ferguson SE, Aronson M, Pollett A, Eiriksson LR, Oza AM, Gallinger S, et al. Performance characteristics of screening strategies for Lynch syndrome in unselected women with newly diagnosed endometrial cancer who have undergone universal germline mutation testing. Cancer. 2014; 120:3932–9.
Article36. Klempner SJ, Fabrizio D, Bane S, Reinhart M, Peoples T, Ali SM, et al. Tumor mutational burden as a predictive biomarker for response to immune checkpoint inhibitors: a review of current evidence. Oncologist. 2020; 25:e147–59.
Article37. Lee SE, Lee MS, Jeon YK, Shim HS, Kang J, Kim J, et al. Interlaboratory comparison study (ring test) of next-generation sequencing-based NTRK fusion detection in South Korea. Cancer Res Treat. 2023; 55:28–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Next generation sequencing and anti-cancer therapy
- What’s new in molecular genetic pathology 2021: solid tumors and NGS panel selection
- Validation of Customized Cancer Panel for Detecting Somatic Mutations and Copy Number Alterations
- Genetic tests by next-generation sequencing in children with developmental delay and/or intellectual disability
- Ultra-rare Disease and Genomics-Driven Precision Medicine