J Korean Med Sci.
2023 Mar;38(12):e91. 10.3346/jkms.2023.38.e91.
Tiotropium Bromide Improves Neutrophilic Asthma by Recovering Histone Deacetylase 2 Activity
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2541039
- DOI: http://doi.org/10.3346/jkms.2023.38.e91
Abstract
- Background
The value of tiotropium bromide (TIO) in neutrophilic asthma was meaningful in previous study. We hypothesized that TIO’s mechanism of action is associated with histone deacetylase 2 (HDAC2) activity, which is key for controlling the transcription of inflammatory cytokines and usually downregulated in neutrophilic asthma.
Methods
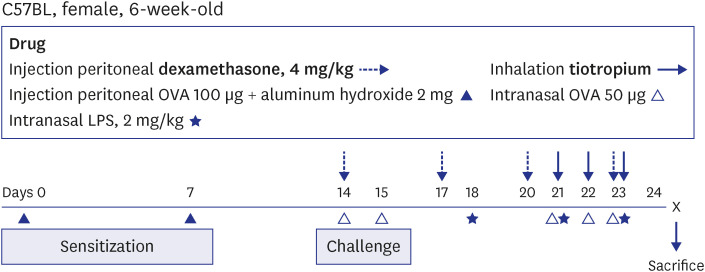
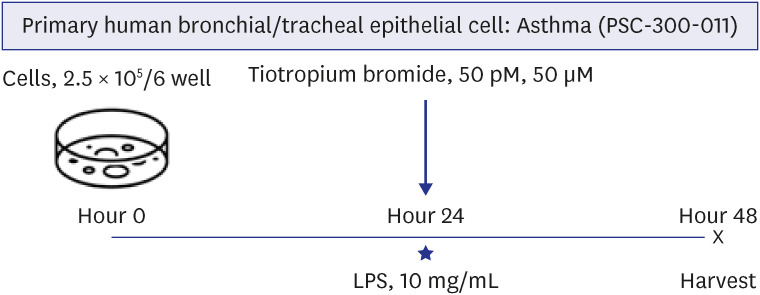
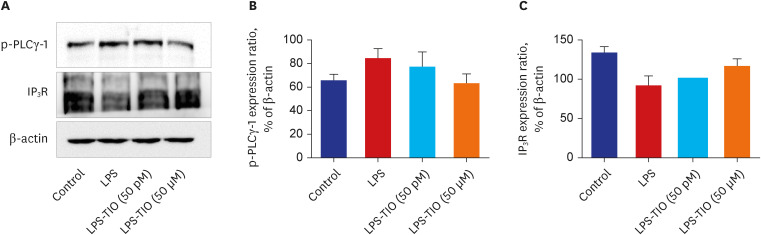
The effects of TIO and dexamethasone (DEX) on HDAC2 activity, nuclear factor kappa B (NF-κB), and C-X-C motif chemokine ligand 1 (CXCL1) were evaluated in neutrophilic asthma mouse model (C57BL, 6-week-old). An in-vitro study was conducted using primary human bronchial/tracheal epithelial (HBE) cells from asthma patients. Western blot analyses were performed for phospho-phospholipase Cγ-1 (PLCγ-1) and inositol trisphosphate (IP3 ) receptors (IP3 R) with treating lipopolysaccharide (LPS) and TIO.
Results
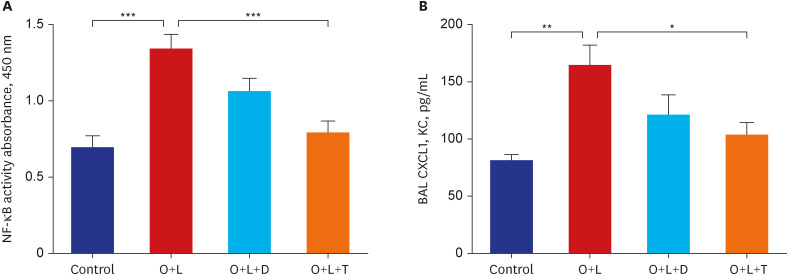
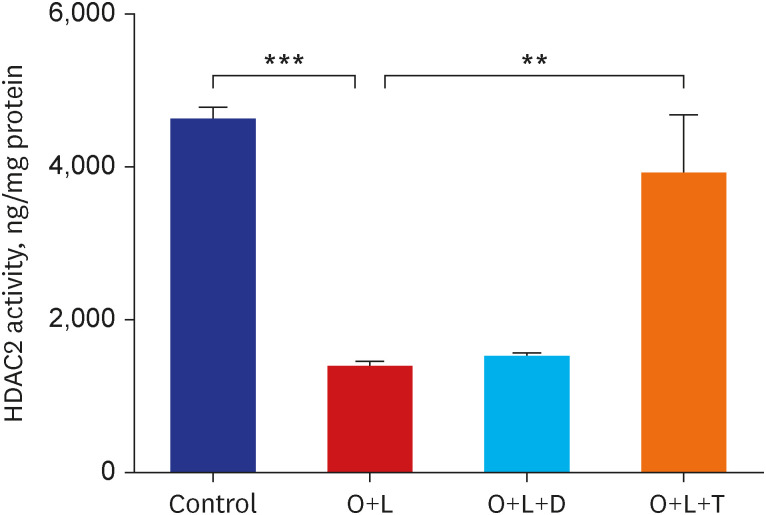
Ovalbumin was used to induce eosinophilic inflammation in this study. After neutrophilic asthma was induced by LPS (O+L group), HDAC2 activity was diminished with increased NF-κB activity and CXCL1 compared to the control group. TIO significantly improved NF-κB activity, CXCL1, and HDAC2 activity compared with the O+L group in in-vivo study (P < 0.05, each). Western blot analyses showed that LPS treated HBE cells from asthma patients increased PLCγ-1 and diminished IP3 receptor levels. After TIO treatment, recovery of IP3 R and improved PLCγ-1 levels were observed.
Conclusion
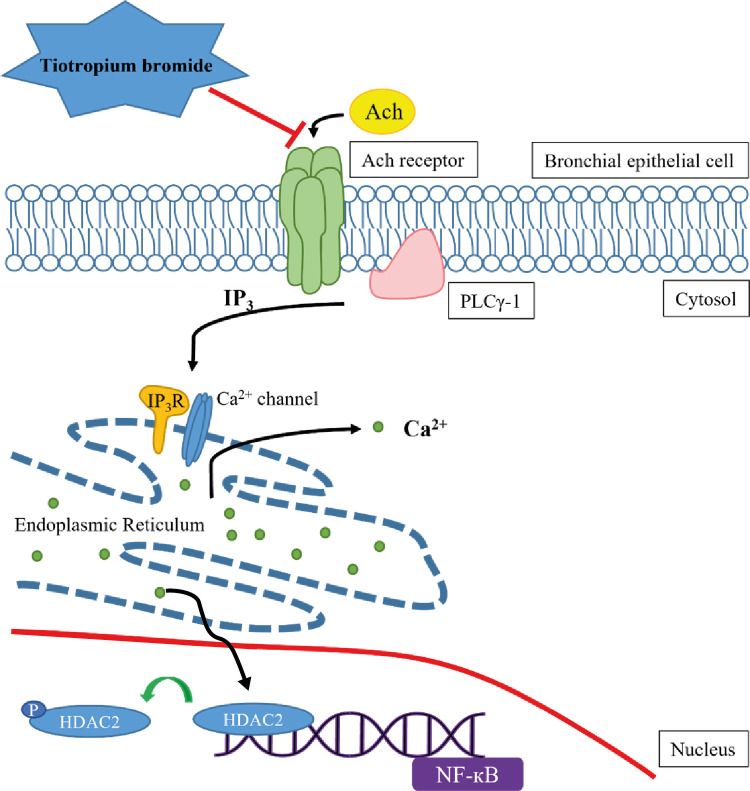
These results support the hypothesis that TIO modulates inflammation by recovering HDAC2 activity from the acetylcholine-stimulated inflammation cascade in neutrophilic asthma. The detailed inflammation cascade of recovering HDAC2 activity by TIO might be associated with PLCγ-1-IP3-IP3R mediated intracellular calcium ion pathway.
Figure
Reference
-
1. Gibson PG, Foster PS. Neutrophilic asthma: welcome back! Eur Respir J. 2019; 54(5):1901846. PMID: 31699782.2. Ray A, Kolls JK. Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol. 2017; 38(12):942–954. PMID: 28784414.3. Kang N, Song WJ. Discovering biomarkers of neutrophilic asthma: a clinician’s perspective. Allergy Asthma Immunol Res. 2022; 14(1):1–4. PMID: 34983102.4. Bullone M, Carriero V, Bertolini F, Folino A, Mannelli A, Di Stefano A, et al. Elevated serum IgE, oral corticosteroid dependence and IL-17/22 expression in highly neutrophilic asthma. Eur Respir J. 2019; 54(5):1900068. PMID: 31439682.5. An TJ, Rhee CK, Kim JH, Lee YR, Chon JY, Park CK, et al. Effects of macrolide and corticosteroid in neutrophilic asthma mouse model. Tuberc Respir Dis (Seoul). 2018; 81(1):80–87. PMID: 29332324.6. Kobayashi Y, Bossley C, Gupta A, Akashi K, Tsartsali L, Mercado N, et al. Passive smoking impairs histone deacetylase-2 in children with severe asthma. Chest. 2014; 145(2):305–312. PMID: 24030221.7. Gunawardhana LP, Gibson PG, Simpson JL, Powell H, Baines KJ. Activity and expression of histone acetylases and deacetylases in inflammatory phenotypes of asthma. Clin Exp Allergy. 2014; 44(1):47–57. PMID: 24355018.
Article8. Lai T, Wu M, Zhang C, Che L, Xu F, Wang Y, et al. HDAC2 attenuates airway inflammation by suppressing IL-17A production in HDM-challenged mice. Am J Physiol Lung Cell Mol Physiol. 2019; 316(1):L269–L279. PMID: 30407865.9. An TJ, Kim JH, Park CK, Yoon HK. Tiotropium bromide has a more potent effect than corticosteroid in the acute neutrophilic asthma mouse model. Tuberc Respir Dis (Seoul). 2022; 85(1):18–24. PMID: 34727490.
Article10. Hamelmann E, Vogelberg C, Szefler SJ. Tiotropium for the treatment of asthma in adolescents. Expert Opin Pharmacother. 2017; 18(3):305–312. PMID: 28110558.
Article11. Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J. 2021; 59(1):2102730. PMID: 34667060.12. Anzalone G, Gagliardo R, Bucchieri F, Albano GD, Siena L, Montalbano AM, et al. IL-17A induces chromatin remodeling promoting IL-8 release in bronchial epithelial cells: effect of Tiotropium. Life Sci. 2016; 152:107–116. PMID: 27038884.
Article13. Wollin L, Pieper MP. Tiotropium bromide exerts anti-inflammatory activity in a cigarette smoke mouse model of COPD. Pulm Pharmacol Ther. 2010; 23(4):345–354. PMID: 20362689.14. Park CK, An TJ, Kim JH, Rhee CK, Yoon HK. Synergistic effect of roflumilast with dexamethasone in a neutrophilic asthma mouse model. Clin Exp Pharmacol Physiol. 2022; 49(6):624–632. PMID: 35181901.
Article15. Holownia A, Mroz RM, Skopinski T, Kielek A, Kolodziejczyk A, Chyczewska E, et al. Tiotropium increases cytosolic muscarinic M3 receptors and acetylated H3 histone proteins in induced sputum cells of COPD patients. Eur J Med Res. 2010; 15(Suppl 2):64–67. PMID: 21147623.16. Neri T, Scalise V, Passalacqua I, Sanguinetti C, Lombardi S, Pergoli L, et al. Tiotropium inhibits proinflammatory microparticle generation by human bronchial and endothelial cells. Sci Rep. 2019; 9(1):11631. PMID: 31406171.17. Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev. 2016; 96(4):1261–1296. PMID: 27512009.18. Kania E, Roest G, Vervliet T, Parys JB, Bultynck G. IP3 receptor-mediated calcium signaling and its role in autophagy in cancer. Front Oncol. 2017; 7:140. PMID: 28725634.19. Kalchiem-Dekel O, Yao X, Levine SJ. Meeting the challenge of identifying new treatments for type 2-low neutrophilic asthma. Chest. 2020; 157(1):26–33. PMID: 31525357.
Article20. Nair P, Surette MG, Virchow JC. Neutrophilic asthma: misconception or misnomer? Lancet Respir Med. 2021; 9(5):441–443. PMID: 33577751.
Article21. Wang M, Gao P, Wu X, Chen Y, Feng Y, Yang Q, et al. Impaired anti-inflammatory action of glucocorticoid in neutrophil from patients with steroid-resistant asthma. Respir Res. 2016; 17(1):153. PMID: 27852250.
Article22. Global Strategy for Asthma Management and Prevention. Updated 2023. Accessed March 4, 2023. www.ginasthma.org .23. Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013; 68(4):322–329. PMID: 23291349.24. Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Efficacy of azithromycin in severe asthma from the AMAZES randomised trial. ERJ Open Res. 2019; 5(4):00056-2019.25. Higham A, Cadden P, Southworth T, Rossall M, Kolsum U, Lea S, et al. Leukotriene B4 levels in sputum from asthma patients. ERJ Open Res. 2016; 2(4):00088-2015. PMID: 28053970.
Article26. Bhatt L, Roinestad K, Van T, Springman EB. Recent advances in clinical development of leukotriene B4 pathway drugs. Semin Immunol. 2017; 33:65–73. PMID: 29042030.27. Wechsler ME, Laviolette M, Rubin AS, Fiterman J, Lapa e Silva JR, Shah PL, et al. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013; 132(6):1295–1302. PMID: 23998657.
Article28. Barnes PJ. Similarities and differences in inflammatory mechanisms of asthma and COPD. Breathe. 2011; 7(3):229–238.
Article29. Barnes PJ. Histone deacetylase-2 and airway disease. Ther Adv Respir Dis. 2009; 3(5):235–243. PMID: 19812111.
Article30. Butler CA, McQuaid S, Taggart CC, Weldon S, Carter R, Skibinski G, et al. Glucocorticoid receptor β and histone deacetylase 1 and 2 expression in the airways of severe asthma. Thorax. 2012; 67(5):392–398. PMID: 22156779.
Article31. Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006; 203(1):7–13. PMID: 16380507.
Article32. Pelaia G, Vatrella A, Busceti MT, Gallelli L, Calabrese C, Terracciano R, et al. Pharmacologic rationale underlying the therapeutic effects of tiotropium/olodaterol in COPD. Ther Clin Risk Manag. 2015; 11:1563–1572. PMID: 26504398.33. Cassambai S, Mee CJ, Renshaw D, Hussain A. Tiotropium bromide, a long acting muscarinic receptor antagonist triggers intracellular calcium signalling in the heart. Toxicol Appl Pharmacol. 2019; 384:114778. PMID: 31618660.34. Saleem H, Tovey SC, Riley AM, Potter BV, Taylor CW. Stimulation of inositol 1,4,5-trisphosphate (IP3) receptor subtypes by adenophostin A and its analogues. PLoS One. 2013; 8(2):e58027. PMID: 23469136.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The role of tiotropium in the management of asthma
- Tiotropium Bromide Has a More Potent Effect Than Corticosteroid in the Acute Neutrophilic Asthma Mouse Model
- Expression of Muscarinic Receptors and the Effect of Tiotropium Bromide in Aged Mouse Model of Chronic Asthma
- A Comparison of International Guidelines for Pediatric Asthma Pharmacotherapy
- Positioning of Long-Acting Muscarinic Antagonists in the Management of Asthma