Ann Pediatr Endocrinol Metab.
2023 Mar;28(1):54-60. 10.6065/apem.2142116.058.
The first case of novel variants of the FSHR mutation causing primary amenorrhea in 2 siblings in Korea
- Affiliations
-
- 1Department of Pediatrics, Pusan National University School of Medicine, Busan, Korea
- 23Billion, Inc., Seoul, Korea
- KMID: 2540788
- DOI: http://doi.org/10.6065/apem.2142116.058
Abstract
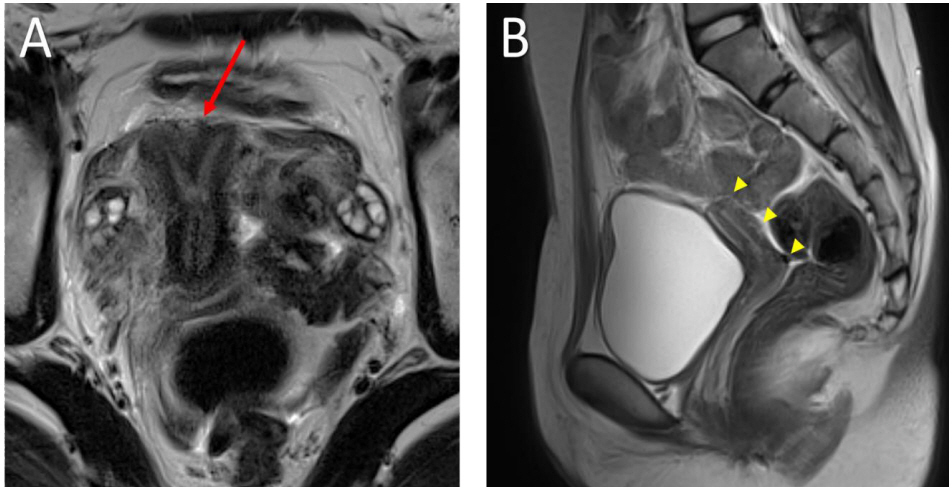
- Follicle-stimulating hormone receptor (FSHR) mutation is a rare cause of amenorrhea. We report the first case of FSHR mutations in Korea. Two female siblings, aged 16 (patient 1) and 19 (patient 2) years, were referred to the pediatric endocrinology clinic because of primary amenorrhea despite normal breast budding. Gonadotropin-releasing hormone stimulation test showed markedly elevated luteinizing hormone and follicle-stimulating hormone with a relatively low level of estrogen, suggesting hypergonadotropic hypogonadism. Pelvic magnetic resonance imaging revealed a bicornuate uterus in patient 1 and uterine hypoplasia with thinning of the endometrium in patient 2. The progesterone challenge test revealed no withdrawal of bleeding. After two months of administration of combined oral contraceptives, menarche was initiated at regular intervals. To determine the genetic cause of amenorrhea in these patients, whole exome sequencing (WES) was performed, which revealed a compound heterozygous FSHR mutation, c.1364T>G (p.Val455Gly) on exon 10, and c.374T>G (p.Leu125Arg) on exon 4; both of which were novel mutations and were confirmed by Sanger sequencing. The patients maintained regular menstruation and improved bone mineral density while taking combined oral contraceptives, calcium, and vitamin D. Therefore, FSHR mutations can be the cause of amenorrhea in Koreans, and WES facilitates diagnosing the rare cause of amenorrhea.
Figure
Reference
-
References
1. Sperling MA. Sperling pediatric endocrinology E-Book. Amsterdam (The Netherlands): Elsevier Health Sciences;2020.2. Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997; 18:739–73.3. Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005; 433:269–77.4. Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010; 120:963–72.5. Ulloa-Aguirre A, Reiter E, Crépieux P. FSH receptor signaling: complexity of interactions and signal diversity. Endocrinology. 2018; 159:3020–35.6. He WB, Du J, Yang XW, Li W, Tang WL, Dai C, et al. Novel inactivating mutations in the FSH receptor cause premature ovarian insufficiency with resistant ovary syndrome. Reprod Biomed Online. 2019; 38:397–406.7. Aittomäki K, Herva R, Stenman UH, Juntunen K, Ylöstalo P, Hovatta O, et al. Clinical features of primary ovarian failure caused by a point mutation in the follicle-stimulating hormone receptor gene. J Clin Endo crinol Metab. 1996; 81:3722–6.8. Shemesh M. Actions of gonadotrophins on the uterus. Reproduction. 2001; 121:835–42.9. Stilley JAW, Segaloff DL. FSH actions and pregnancy: looking beyond ovarian FSH receptors. Endocrinology. 2018; 159:4033–42.10. Danilovich N, Roy I, Sairam MRJE. Emergence of uterine pathology during accelerated biological aging in FSH receptor-haploinsufficient mice. Endocrinology. 2002; 143:3618–27.11. Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, et al. FSH directly regulates bone mass. Cell. 2006; 125:247–60.12. Kim NK, Lee SH, Nam YS, Sohn TJ, Park SH, Park C, et al. Molecular variants of the FSH receptor exon 10 (Thr307Ala; A919G) in premature ovarian failure (POF) women by PCR-SSCP. Korean J Obstet Gynecol. 2000; 43:1144–6.13. Choi YM, Kim SH, Kim JK, Moon SY, Lee JY, Lee GW. Follicle stimulating hormone receptor gene mutation in Korean women with premature ovarian failure and normal karyotype. Korean J Obstet Gynecol. 2001; 43:836–41.14. Nam Y, Kim N, Choi M, Park S, Chung K, Lee S, et al. Analysis of follicle stimulating hormone receptor gene mutation in Korean. Clin Exp Reprod Med. 1998; 25:281–6.15. França MM, Lerario AM, Funari MFA, Nishi MY, Narcizo AM, de Mello MP, et al. A novel homozygous missense FSHR variant associated with hypergonadotropic hypogonadism in two siblings from a Brazilian family. Sex Dev. 2017; 11:137–42.16. Liu H, Xu X, Han T, Yan L, Cheng L, Qin Y, et al. A novel homozygous mutation in the FSHR gene is causative for primary ovarian insufficiency. Fertil Steril. 2017; 108:1050–5.e2.17. Bramble MS, Goldstein EH, Lipson A, Ngun T, Eskin A, Gosschalk JE, et al. A novel follicle-stimulating hormone receptor mutation causing primary ovarian failure: a fertility application of whole exome sequencing. Hum Reprod. 2016; 31:905–14.18. Katari S, Wood-Trageser MA, Jiang H, Kalynchuk E, Muzumdar R, Yatsenko SA, et al. Novel Inactivating Mutation of the FSH Receptor in Two Siblings of Indian Origin With Premature Ovarian Failure. J Clin Endocrinol Metab. 2015; 100:2154–7.19. Achrekar SK, Modi DN, Meherji PK, Patel ZM, Mahale SD. Follicle stimulating hormone receptor gene variants in women with primary and secondary amenorrhea. J Assist Reprod Genet. 2010; 27:317–26.20. Kuechler A, Hauffa BP, Köninger A, Kleinau G, Albrecht B, Horsthemke B, et al. An unbalanced translocation unmasks a recessive mutation in the follicle-stimulating hormone receptor (FSHR) gene and causes FSH resistance. Eur J Hum Genet. 2010; 18:656–61.21. Nakamura Y, Maekawa R, Yamagata Y, Tamura I, Sugino N. A novel mutation in exon8 of the follicle-stimulating hormone receptor in a woman with primary amenorrhea. Gynecol Endocrinol. 2008; 24:708–12.22. Allen LA, Achermann JC, Pakarinen P, Kotlar TJ, Huhtaniemi IT, Jameson JL, et al. A novel loss of function mutation in exon 10 of the FSH receptor gene causing hypergonadotrophic hypogonadism: clinical and molecular characteristics. Hum Reprod. 2003; 18:251–6.23. Meduri G, Touraine P, Beau I, Lahuna O, Desroches A, Vacher-Lavenu MC, et al. Delayed puberty and primary amenorrhea associated with a novel mutation of the human follicle-stimulating hormone receptor: clinical, histological, and molecular studies. J Clin Endocrinol Metab. 2003; 88:3491–8.24. Doherty E, Pakarinen P, Tiitinen A, Kiilavuori A, Huhtaniemi I, Forrest S, et al. A novel mutation in the FSH receptor inhibiting signal transduction and causing primary ovarian failure. J Clin Endocrinol Metab. 2002; 87:1151–5.25. Touraine P, Beau I, Gougeon A, Meduri G, Desroches A, Pichard C, et al. New natural inactivating mutations of the follicle-stimulating hormone receptor: correlations between receptor function and phenotype. Mol Endocrinol. 1999; 13:1844–54.26. Beau I, Touraine P, Meduri G, Gougeon A, Desroches A, Matuchansky C, et al. A novel phenotype related to partial loss of function mutations of the follicle stimulating hormone receptor. J Clin Invest. 1998; 102:1352–9.27. Gromoll J, Simoni M, Nordhoff V, Behre HM, De Geyter C, Nieschlag E. Functional and clinical consequences of mutations in the FSH receptor. Mol Cell Endocrinol. 1996; 125:177–82.28. Aittomäki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995; 82:959–68.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of 46XX, Primary Amenorrhea, Absent Gonads and Lack of Mullerian Ducts
- A Cytogenetic Study of Amenorrhea
- Evaluation and management of amenorrhea related to congenital sex hormonal disorders
- Cytogenetic Analysis for Amenorrhea
- A Case of Hypogonadotrophic Hypogonadism due to Intrasellar Arachnoid Cyst