J Korean Med Sci.
2023 Mar;38(11):e85. 10.3346/jkms.2023.38.e85.
Comparison of Metastatic Patterns Among Neuroendocrine Tumors, Neuroendocrine Carcinomas, and Nonneuroendocrine Carcinomas of Various Primary Organs
- Affiliations
-
- 1Department of Pathology, Chungnam National University School of Medicine, Daejeon, Korea
- 2Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2540651
- DOI: http://doi.org/10.3346/jkms.2023.38.e85
Abstract
- Background
Both neuroendocrine tumors (NETs) and neuroendocrine carcinomas (NECs) exhibit neuroendocrine differentiation and are classified as neuroendocrine neoplasms (NENs). NECs and nonneuroendocrine neoplasms (non-NENs), such as adenocarcinoma, have similar mutational profiles. The purpose of this study was to identify differences in metastatic patterns and to identify the key factor causing these differences by simultaneously comparing the metastatic patterns of NETs, NECs and non-NENs from various primary organs.
Methods
We retrieved data for 4,223 patients with NENs and 41,637 patients with non-NENs arising at various primary sites from an institutional database and then compared NET, NEC, and non-NEN metastatic patterns.
Results
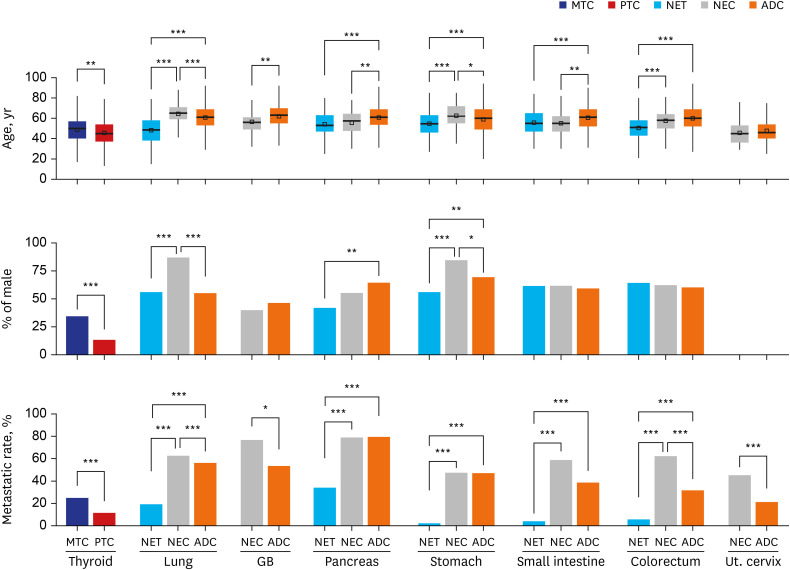
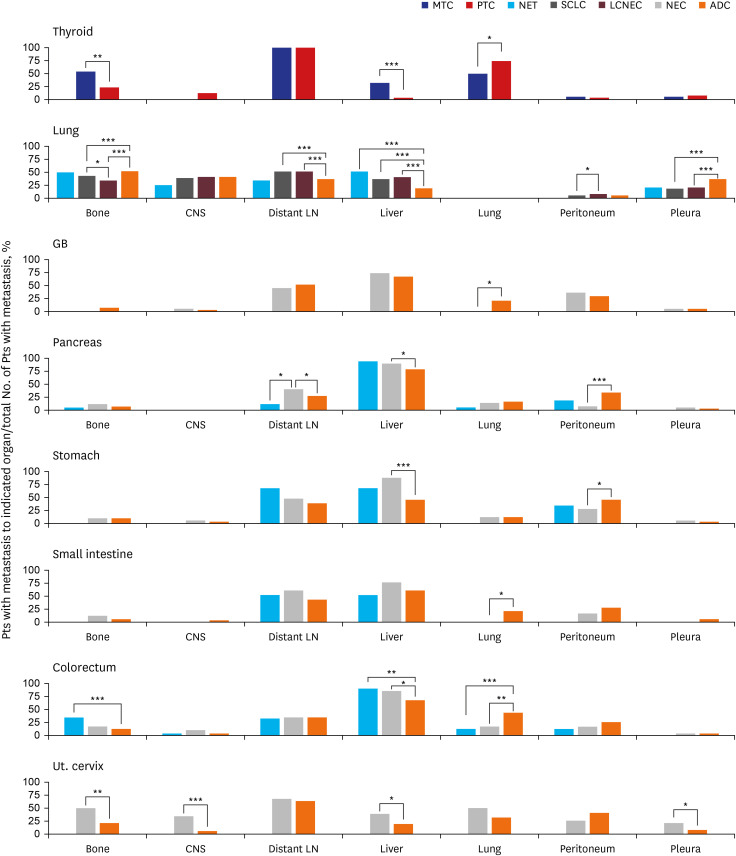
NETs and NECs showed generally similar metastatic patterns. Most NEN patients had a higher liver organotrophic metastasis rate, lower lung organotrophic metastasis rate, and lower pleural/peritoneal organotrophic metastasis rate than non-NEN patients. Some differences were characteristics of specific organs. Some of these site-specific differences were not caused by NENs but by non-NENs, including a higher bone organotrophic metastasis rate for medullary thyroid carcinoma and a lower bone organotrophic metastasis rate for pulmonary NEN. Other differences were probably caused by NENs, including a higher bone organotrophic metastasis rate for colorectal NETs. Uterine cervical NEC showed unique patterns of metastasis compared to NEN from other sites.
Conclusion
Significant differences between the metastatic patterns of NENs and non-NENs were detected. The multigene program that causes neuroendocrine differentiation might be associated with organotropic metastasis.
Figure
Reference
-
1. Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018; 31(12):1770–1786. PMID: 30140036.2. Zheng Z, Chen C, Jiang L, Zhou X, Dai X, Song Y, et al. Incidence and risk factors of gastrointestinal neuroendocrine neoplasm metastasis in liver, lung, bone, and brain: a population-based study. Cancer Med. 2019; 8(17):7288–7298. PMID: 31609098.3. Cai W, Tan Y, Ge W, Ding K, Hu H. Pattern and risk factors for distant metastases in gastrointestinal neuroendocrine neoplasms: a population-based study. Cancer Med. 2018; 7(6):2699–2709. PMID: 29733523.4. Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer. 2016; 139(12):2679–2686. PMID: 27553864.5. Wang S, Zhang J, Liu S, Zhang J. The prognostic analysis of different metastatic patterns in pancreatic neuroendocrine tumors patients: a population based analysis. Medicine (Baltimore). 2019; 98(44):e17773. PMID: 31689842.6. Hermans BC, de Vos-Geelen J, Derks JL, Latten L, Liem IH, van der Zwan JM, et al. Unique metastatic patterns in neuroendocrine neoplasms of different primary origin. Neuroendocrinology. 2021; 111(11):1111–1120. PMID: 33227805.7. Takizawa N, Ohishi Y, Hirahashi M, Takahashi S, Nakamura K, Tanaka M, et al. Molecular characteristics of colorectal neuroendocrine carcinoma; similarities with adenocarcinoma rather than neuroendocrine tumor. Hum Pathol. 2015; 46(12):1890–1900. PMID: 26434631.8. Konukiewitz B, Jesinghaus M, Steiger K, Schlitter AM, Kasajima A, Sipos B, et al. Pancreatic neuroendocrine carcinomas reveal a closer relationship to ductal adenocarcinomas than to neuroendocrine tumors G3. Hum Pathol. 2018; 77:70–79. PMID: 29596894.9. Lee YM, Yeo MK, Choi SY, Kim KH, Suh KS. Peritoneal fluid cytology of disseminated large cell neuroendocrine carcinoma combined with endometrioid adenocarcinoma of the endometrium. J Pathol Transl Med. 2019; 53(6):407–410. PMID: 31525833.10. Bae HI, Lee C, Jo YM, Kwon O, Yu W, Kim MS, et al. Gastric mixed adenoneuroendocrine carcinoma with squamous differentiation: a case report. J Pathol Transl Med. 2016; 50(4):318–321. PMID: 26755357.11. Jung J, Chae YS, Kim CH, Lee Y, Lee JH, Kim DS, et al. Combined adenosquamous and large cell neuroendocrine carcinoma of the gallbladder. J Pathol Transl Med. 2018; 52(2):121–125. PMID: 28994275.12. Kwon HJ, Kim JW, Kim H, Choi Y, Ahn S. Combined hepatocellular carcinoma and neuroendocrine carcinoma with ectopic secretion of parathyroid hormone: a case report and review of the literature. J Pathol Transl Med. 2018; 52(4):232–237. PMID: 29794961.13. Lee SW, Lee IS, Cho YK, Park JM, Kim SW, Choi MG, et al. A case of mixed adenoneuroendocrine carcinoma of the common bile duct: initially diagnosed as cholangiocarcinoma. Korean J Pathol. 2014; 48(6):445–448. PMID: 25588638.14. Farooq F, Zarrabi K, Sweeney K, Kim J, Bandovic J, Patel C, et al. Multiregion comprehensive genomic profiling of a gastric mixed neuroendocrine-nonneuroendocrine neoplasm with trilineage differentiation. J Gastric Cancer. 2018; 18(2):200–207. PMID: 29984070.15. Gastrointestinal Pathology Study Group of Korean Society of Pathologists. Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, et al. Current trends of the incidence and pathological diagnosis of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea 2000-2009: multicenter study. Cancer Res Treat. 2012; 44(3):157–165. PMID: 23091441.16. Song L, Zhai X, Yu S, Ma Y, Wang F, Yu X, et al. Clinical analysis of 547 patients with neuroendocrine tumors in a Chinese population: a single-center study. Cancer Med. 2019; 8(8):3729–3737. PMID: 31127690.17. Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010; 45(2):234–243. PMID: 20058030.18. Kim HK, Nam SJ. Management of T1 Rectal NEN. J Dig Cancer Rep. 2019; 7(2):45–50.19. Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004; 240(1):117–122. PMID: 15213627.20. Goksu SY, Ozer M, Beg MS, Sanford NN, Ahn C, Fangman BD, et al. Racial/ethnic disparities and survival characteristics in non-pancreatic gastrointestinal tract neuroendocrine tumors. Cancers (Basel). 2020; 12(10):2990. PMID: 33076486.21. Ronot M, Clift AK, Baum RP, Singh A, Kulkarni HR, Frilling A, et al. Morphological and functional imaging for detecting and assessing the resectability of neuroendocrine liver metastases. Neuroendocrinology. 2018; 106(1):74–88. PMID: 28728155.22. Gibson WE, Gonzalez RS, Cates JM, Liu E, Shi C. Hepatic micrometastases are associated with poor prognosis in patients with liver metastases from neuroendocrine tumors of the digestive tract. Hum Pathol. 2018; 79:109–115. PMID: 29763717.23. Pea A, Yu J, Marchionni L, Noe M, Luchini C, Pulvirenti A, et al. Genetic analysis of small well-differentiated pancreatic neuroendocrine tumors identifies subgroups with differing risks of liver metastases. Ann Surg. 2020; 271(3):566–573. PMID: 30339629.24. Huang Q, Wu H, Nie L, Shi J, Lebenthal A, Chen J, et al. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013; 37(4):467–483. PMID: 23426118.25. Hugen N, Sloot YJ, Netea-Maier RT, van de Water C, Smit JW, Nagtegaal ID, et al. Divergent metastatic patterns between subtypes of thyroid carcinoma results from the nationwide Dutch pathology registry. J Clin Endocrinol Metab. 2020; 105(3):e299–e306. PMID: 31641763.26. Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014; 86(1):78–84. PMID: 25130083.27. Park HK. Neuroendocrine carcinomas of the uterine cervix, endometrium, and ovary show higher tendencies for bone, brain, and liver organotrophic metastases. Curr Oncol. 2022; 29(10):7461–7469. PMID: 36290864.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mammographic, Sonographic, and MRI Features of Primary Neuroendocrine Carcinoma of the Breast: A Case Report
- Skin Metastasis of Neuroendocrine Carcinoma Arising in the Rectum
- Multiregion Comprehensive Genomic Profiling of a Gastric Mixed Neuroendocrine-Nonneuroendocrine Neoplasm with Trilineage Differentiation
- Primary Large Cell Neuroendocrine Carcinoma of the Breast: Radiologic and Pathologic Findings
- Two Cases of Neuroendocrine Carcinomas of the Stomach: Large Cell Carcinoma and Small Cell Carcinoma