Arch Hand Microsurg.
2023 Mar;28(1):63-66. 10.12790/ahm.22.0057.
Cefepime-induced neurotoxicity after flap surgery: a rare case report
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Soonchunhyang University Seoul Hospital, Seoul, Korea
- 2Department of Plastic and Reconstructive Surgery, Soonchunhyang University Cheonan Hospital, Cheonan, Korea
- 3Institute of Tissue Regeneration, Soonchunhyang University College of Medicine, Cheonan, Korea
- KMID: 2540034
- DOI: http://doi.org/10.12790/ahm.22.0057
Abstract
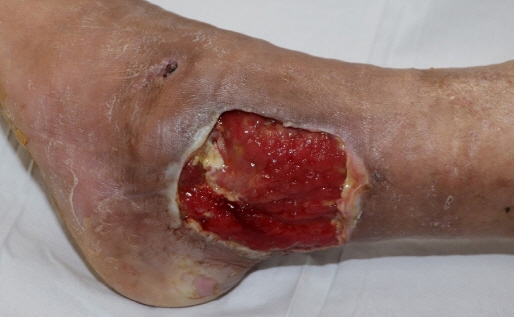
- Cefepime is a fourth-generation cephalosporin that covers gram-positive bacteria and gram-negative bacteria, such as Pseudomonas. A 48-year-old male patient underwent a posterior tibial artery perforator-based fasciocutaneous turnover flap and was administered cefepime. After 2 days, the patient showed neurological symptoms, such as cognitive decline and aphasia. We immediately stopped cefepime and changed to ciprofloxacin. In addition, thiamine was administered and additional dialysis was performed. The neurological symptoms were resolved after tapering cefepime and hemodialysis. In patients undergoing flap surgery, especially those with impaired renal function, cefepime should be administered carefully considering the risk of neurotoxicity.
Figure
Reference
-
References
1. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013; 14:73–156.2. Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis. 2007; 7:338–48.3. Payne LE, Gagnon DJ, Riker RR, et al. Cefepime-induced neurotoxicity: a systematic review. Crit Care. 2017; 21:276.4. Drago L, De Vecchi E. The safety of cefepime in the treatment of infection. Expert Opin Drug Saf. 2008; 7:377–87.5. Li HT, Lee CH, Wu T, et al. Clinical, Electroencephalographic features and prognostic factors of cefepime-induced neurotoxicity: a retrospective study. Neurocrit Care. 2019; 31:329–37.6. Boschung-Pasquier L, Atkinson A, Kastner LK, et al. Cefepime neurotoxicity: thresholds and risk factors: a retrospective cohort study. Clin Microbiol Infect. 2020; 26:333–9.7. Kwon J, Choi JY, Bae EK. Cefepime-induced aphasic status epilepticus mimicking acute stroke. J Epilepsy Res. 2014; 4:85–7.8. Glicksman A, Ferder M, Casale P, Posner J, Kim R, Strauch B. 1457 years of microsurgical experience. Plast Reconstr Surg. 1997; 100:355–63.9. Bausch S, Araschmid LJ, Hardmeier M, Osthoff M. Cefepime-induced neurotoxicity in the setting of acute kidney injury: a case series and discussion of preventive measures. Cureus. 2022; 14:e26392.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cefepime Neurotoxicity in Patients with Renal Insufficiency
- Cefepime-induced neurotoxicity
- Neurotoxicity Induced by Cefepime in a Patient with Minimal Change Disease
- Cefepime-Induced Reversible Encephalopathy with Triphasic Waves in Patients with Impaired Renal Function
- Cefepime-induced nonconvulsive status epilepticus in a hemodialysis patient