Korean J Physiol Pharmacol.
2023 Mar;27(2):143-155. 10.4196/kjpp.2023.27.2.143.
Resveratrol pretreatment alleviates NLRP3 inflammasomemediated cardiomyocyte pyroptosis by targeting TLR4/MyD88/ NF-κB signaling cascade in coronary microembolization-induced myocardial damage
- Affiliations
-
- 1Department of Cardiology, The First Affiliated Hospital of Guangxi Medical University & Guangxi Key Laboratory Base of Precision Medicine in Cardio- cerebrovascular Diseases Control and Prevention & Guangxi Clinical Research Center for Cardio-cerebrovascular Diseases, Nanning 530021, China
- 2Department of Cardiology, Affiliated Liutie Central Hospital of Guangxi Medical University, Liuzhou 545007, China
- KMID: 2539569
- DOI: http://doi.org/10.4196/kjpp.2023.27.2.143
Abstract
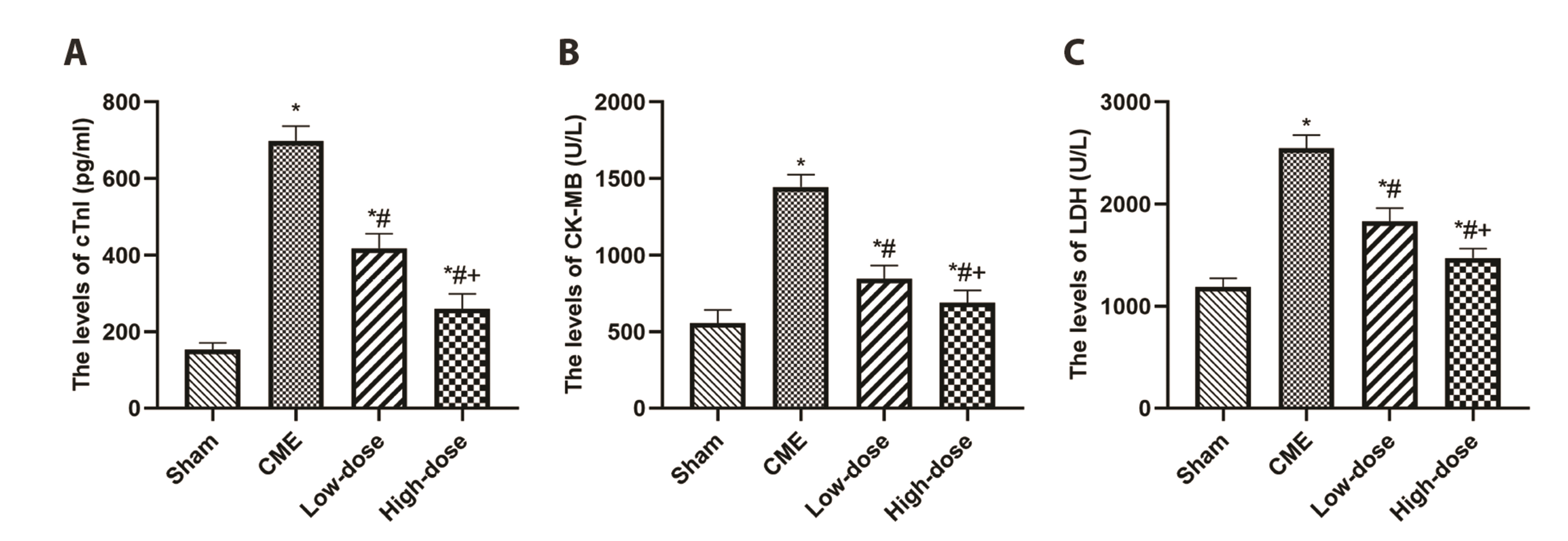
- Percutaneous coronary intervention and acute coronary syndrome are both closely tied to the frequently occurring complication of coronary microembolization (CME). Resveratrol (RES) has been shown to have a substantial cardioprotective influence in a variety of cardiac diseases, though its function and potential mechanistic involvement in CME are still unclear. The forty Sprague–Dawley rats were divided into four groups randomly: CME, CME + RES (25 mg/kg), CME + RES (50 mg/kg), and sham (10 rats per group). The CME model was developed. Echocardiography, levels of myocardial injury markers in the serum, and histopathology of the myocardium were used to assess the function of the cardiac muscle. For the detection of the signaling of TLR4/MyD88/NF-κB along with the expression of pyroptosisrelated molecules, ELISA, qRT-PCR, immunofluorescence, and Western blotting were used, among other techniques. The findings revealed that myocardial injury and pyroptosis occurred in the myocardium following CME, with a decreased function of cardiac, increased levels of serum myocardial injury markers, increased area of microinfarct, as well as a rise in the expression levels of pyroptosis-related molecules. In addition to this, pretreatment with resveratrol reduced the severity of myocardial injury after CME by improving cardiac dysfunction, decreasing serum myocardial injury markers, decreasing microinfarct area, and decreasing cardiomyocyte pyroptosis, primarily by blocking the signaling of TLR4/MyD88/NF-κB and also reducing the NLRP3 inflammasome activation. Resveratrol may be able to alleviate CME-induced myocardial pyroptosis and cardiac dysfunction by impeding the activation of NLRP3 inflammasome and the signaling pathway of TLR4/MyD88/NF-κB.
Figure
Reference
-
1. Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, Schulz R, Erbel R. 2009; Coronary microembolization: from bedside to bench and back to bedside. Circulation. 120:1822–1836. DOI: 10.1161/CIRCULATIONAHA.109.888784. PMID: 19884481. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70449514842&origin=inward.2. Bahrmann P, Werner GS, Heusch G, Ferrari M, Poerner TC, Voss A, Figulla HR. 2007; Detection of coronary microembolization by Doppler ultrasound in patients with stable angina pectoris undergoing elective percutaneous coronary interventions. Circulation. 115:600–608. DOI: 10.1161/CIRCULATIONAHA.106.660779. PMID: 17261655. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33846864001&origin=inward.
Article3. Su Q, Li L, Zhao J, Sun Y, Yang H. 2017; MiRNA expression profile of the myocardial tissue of pigs with coronary microembolization. Cell Physiol Biochem. 43:1012–1024. DOI: 10.1159/000481699. PMID: 28968594. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030721654&origin=inward.
Article4. Su Q, Li L, Wang J, Zhou Y, Liu Y. 2015; Mechanism of programmed cell death factor 4/nuclear factor-κB signaling pathway in porcine coronary micro-embolization-induced cardiac dysfunction. Exp Biol Med (Maywood). 240:1426–1433. DOI: 10.1177/1535370215573400. PMID: 25769315. PMCID: PMC4935293. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84944102029&origin=inward.
Article5. Thielmann M, Dörge H, Martin C, Belosjorow S, Schwanke U, van De Sand A, Konietzka I, Büchert A, Krüger A, Schulz R, Heusch G. 2002; Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res. 90:807–813. DOI: 10.1161/01.RES.0000014451.75415.36. PMID: 11964374. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037134405&origin=inward.6. Heusch G. 2016; The coronary circulation as a target of cardioprotection. Circ Res. 118:1643–1658. DOI: 10.1161/CIRCRESAHA.116.308640. PMID: 27174955. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84968764909&origin=inward.
Article7. Heusch G, Gersh BJ. 2017; The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J. 38:774–784. DOI: 10.1093/eurheartj/ehw224. PMID: 27354052. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85018173958&origin=inward.
Article8. Samali A, Zhivotovsky B, Jones D, Nagata S, Orrenius S. 1999; Apoptosis: cell death defined by caspase activation. Cell Death Differ. 6:495–496. DOI: 10.1038/sj.cdd.4400520. PMID: 10381647. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033047234&origin=inward.
Article9. Bergsbaken T, Fink SL, Cookson BT. 2009; Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 7:99–109. DOI: 10.1038/nrmicro2070. PMID: 19148178. PMCID: PMC2910423. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=58449083290&origin=inward.
Article10. Lei Q, Yi T, Chen C. 2018; NF-κB-gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monit. 24:6044–6052. DOI: 10.12659/MSM.908529. PMID: 30161099. PMCID: PMC6128186. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85053683163&origin=inward.
Article11. Zeng C, Duan F, Hu J, Luo B, Huang B, Lou X, Sun X, Li H, Zhang X, Yin S, Tan H. 2020; NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 34:101523. DOI: 10.1016/j.redox.2020.101523. PMID: 32273259. PMCID: PMC7327979. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082654900&origin=inward.
Article12. Zhaolin Z, Guohua L, Shiyuan W, Zuo W. 2019; Role of pyroptosis in cardiovascular disease. Cell Prolif. 52:e12563. DOI: 10.1111/cpr.12563. PMID: 30525268. PMCID: PMC6496801. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85058158091&origin=inward.
Article13. Lundberg AM, Ketelhuth DF, Johansson ME, Gerdes N, Liu S, Yamamoto M, Akira S, Hansson GK. 2013; Toll-like receptor 3 and 4 signalling through the TRIF and TRAM adaptors in haematopoietic cells promotes atherosclerosis. Cardiovasc Res. 99:364–373. DOI: 10.1093/cvr/cvt033. PMID: 23417039. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84878805816&origin=inward.
Article14. Lu M, Tang F, Zhang J, Luan A, Mei M, Xu C, Zhang S, Wang H, Maslov LN. 2015; Astragaloside IV attenuates injury caused by myocardial ischemia/reperfusion in rats via regulation of toll-like receptor 4/nuclear factor-κB signaling pathway. Phytother Res. 29:599–606. DOI: 10.1002/ptr.5297. PMID: 25604645. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84926482662&origin=inward.
Article15. Soraya H, Clanachan AS, Rameshrad M, Maleki-Dizaji N, Ghazi-Khansari M, Garjani A. 2014; Chronic treatment with metformin suppresses toll-like receptor 4 signaling and attenuates left ventricular dysfunction following myocardial infarction. Eur J Pharmacol. 737:77–84. DOI: 10.1016/j.ejphar.2014.05.003. PMID: 24842192. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84901733607&origin=inward.
Article16. Chimenti C, Verardo R, Scopelliti F, Grande C, Petrosillo N, Piselli P, De Paulis R, Frustaci A. 2017; Myocardial expression of Toll-like receptor 4 predicts the response to immunosuppressive therapy in patients with virus-negative chronic inflammatory cardiomyopathy. Eur J Heart Fail. 19:915–925. DOI: 10.1002/ejhf.796. PMID: 28370906. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85017335618&origin=inward.
Article17. Ma SR, Xie XW. 2017; NLRC5 deficiency promotes myocardial damage induced by high fat diet in mice through activating TLR4/NF-κB. Biomed Pharmacother. 91:755–766. DOI: 10.1016/j.biopha.2017.03.062. PMID: 28499247. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85019027509&origin=inward.
Article18. Zhang J, Zhang J, Yu P, Chen M, Peng Q, Wang Z, Dong N. 2017; Remote ischaemic preconditioning and sevoflurane postconditioning synergistically protect rats from myocardial injury induced by ischemia and reperfusion partly via inhibition TLR4/MyD88/NF-κB signaling pathway. Cell Physiol Biochem. 41:22–32. DOI: 10.1159/000455815. PMID: 28135708. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85026460153&origin=inward.
Article19. Dörge H, Schulz R, Belosjorow S, Post H, van de Sand A, Konietzka I, Frede S, Hartung T, Vinten-Johansen J, Youker KA, Entman ML, Erbel R, Heusch G. 2002; Coronary microembolization: the role of TNF-alpha in contractile dysfunction. J Mol Cell Cardiol. 34:51–62. DOI: 10.1006/jmcc.2001.1489. PMID: 11812164. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036172194&origin=inward.20. Su Q, Li L, Sun Y, Yang H, Ye Z, Zhao J. 2018; Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3 inflammasome in coronary microembolization-induced myocardial injury. Cell Physiol Biochem. 47:1497–1508. DOI: 10.1159/000490866. PMID: 29940584. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85050792078&origin=inward.
Article21. Wang XT, Lu YX, Sun YH, He WK, Liang JB, Li L. 2017; TAK-242 protects against apoptosis in coronary microembolization-induced myocardial injury in rats by suppressing TLR4/NF-κB signaling pathway. Cell Physiol Biochem. 41:1675–1683. DOI: 10.1159/000471248. PMID: 28359050. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85016550381&origin=inward.
Article22. Wiciński M, Socha M, Walczak M, Wódkiewicz E, Malinowski B, Rewerski S, Górski K, Pawlak-Osińska K. 2018; Beneficial effects of resveratrol administration-focus on potential biochemical mechanisms in cardiovascular conditions. Nutrients. 10:1813. DOI: 10.3390/nu10111813. PMID: 30469326. PMCID: PMC6266814. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85057100327&origin=inward.
Article23. Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. 2010; Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 299:H18–H24. DOI: 10.1152/ajpheart.00260.2010. PMID: 20418481. PMCID: PMC2904129. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77953741269&origin=inward.
Article24. Yang Y, Wang X, Zhang L, An H, Zao Z. 2011; Inhibitory effects of resveratrol on platelet activation induced by thromboxane A2 receptor agonist in human platelets. Am J Chin Med. 39:145–159. DOI: 10.1142/S0192415X11008713. PMID: 21213405. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78751525495&origin=inward.
Article25. Abbas AM. 2016; Cardioprotective effect of resveratrol analogue isorhapontigenin versus omega-3 fatty acids in isoproterenol-induced myocardial infarction in rats. J Physiol Biochem. 72:469–484. DOI: 10.1007/s13105-016-0494-4. PMID: 27193109. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84969760158&origin=inward.
Article26. Xu K, Liu XF, Ke ZQ, Yao Q, Guo S, Liu C. 2018; Resveratrol modulates apoptosis and autophagy induced by high glucose and palmitate in cardiac cells. Cell Physiol Biochem. 46:2031–2040. DOI: 10.1159/000489442. PMID: 29723857. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85046474193&origin=inward.
Article27. Yang L, Zhang Y, Zhu M, Zhang Q, Wang X, Wang Y, Zhang J, Li J, Yang L, Liu J, Liu F, Yang Y, Kang L, Shen Y, Qi Z. 2016; Resveratrol attenuates myocardial ischemia/reperfusion injury through up-regulation of vascular endothelial growth factor B. Free Radic Biol Med. 101:1–9. DOI: 10.1016/j.freeradbiomed.2016.09.016. PMID: 27667182. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84989824817&origin=inward.
Article28. Li J, Xie C, Zhuang J, Li H, Yao Y, Shao C, Wang H. 2015; Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: role of the TLR4/NF-κB signaling pathway. Mol Med Rep. 11:1120–1126. DOI: 10.3892/mmr.2014.2955. PMID: 25405531. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84916239900&origin=inward.
Article29. Feng H, Mou SQ, Li WJ, Zhang N, Zhou ZY, Ding W, Bian ZY, Liao HH. 2020; Resveratrol inhibits ischemia-induced myocardial senescence signals and NLRP3 inflammasome activation. Oxid Med Cell Longev. 2020:2647807. DOI: 10.1155/2020/2647807. PMID: 32908628. PMCID: PMC7468658. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85090820609&origin=inward.
Article30. Hong SW, Jung KH, Zheng HM, Lee HS, Suh JK, Park IS, Lee DH, Hong SS. 2010; The protective effect of resveratrol on dimethylnitrosamine-induced liver fibrosis in rats. Arch Pharm Res. 33:601–609. DOI: 10.1007/s12272-010-0415-y. PMID: 20422370. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77954544210&origin=inward.
Article31. Mao Q, Liang X, Wu Y, Lu Y. 2019; Resveratrol attenuates cardiomyocyte apoptosis in rats induced by coronary microembolization through SIRT1-mediated deacetylation of p53. J Cardiovasc Pharmacol Ther. 24:551–558. DOI: 10.1177/1074248419845916. PMID: 31046448. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85065450832&origin=inward.
Article32. Su Q, Lv X, Sun Y, Ye Z, Kong B, Qin Z. 2018; Role of TLR4/MyD88/NF-κB signaling pathway in coronary microembolization-induced myocardial injury prevented and treated with nicorandil. Biomed Pharmacother. 106:776–784. DOI: 10.1016/j.biopha.2018.07.014. PMID: 29990871. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85049466723&origin=inward.
Article33. Dörge H, Neumann T, Behrends M, Skyschally A, Schulz R, Kasper C, Erbel R, Heusch G. 2000; Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol. 279:H2587–H2592. DOI: 10.1152/ajpheart.2000.279.6.H2587. PMID: 11087208. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034530272&origin=inward.
Article34. Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu C, Duan J. 2017; The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3 inflammasome pathway. Biomed Pharmacother. 91:1042–1052. DOI: 10.1016/j.biopha.2017.05.033. PMID: 28525945. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85019256033&origin=inward.
Article35. Nakata R, Takahashi S, Inoue H. 2012; Recent advances in the study on resveratrol. Biol Pharm Bull. 35:273–279. DOI: 10.1248/bpb.35.273. PMID: 22382311. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84857831235&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tanshinone IIA reduces pyroptosis in rats with coronary microembolization by inhibiting the TLR4/MyD88/NF-κB/NLRP3 pathway
- The role of discoid domain receptor 1 on renal tubular epithelial pyroptosis in diabetic nephropathy
- Chronic cold stress-induced myocardial injury: effects on oxidative stress, inflammation and pyroptosis
- Puerarin pretreatment attenuates cardiomyocyte apoptosis induced by coronary microembolization in rats by activating the PI3K/Akt/GSK-3β signaling pathway
- MiR-182-5p Mediated by Exosomes Derived From Bone Marrow Mesenchymal Stem Cell Attenuates Inflammatory Responses by Targeting TLR4 in a Mouse Model of Myocardial Infraction