Korean J Physiol Pharmacol.
2021 Mar;25(2):147-157. 10.4196/kjpp.2021.25.2.147.
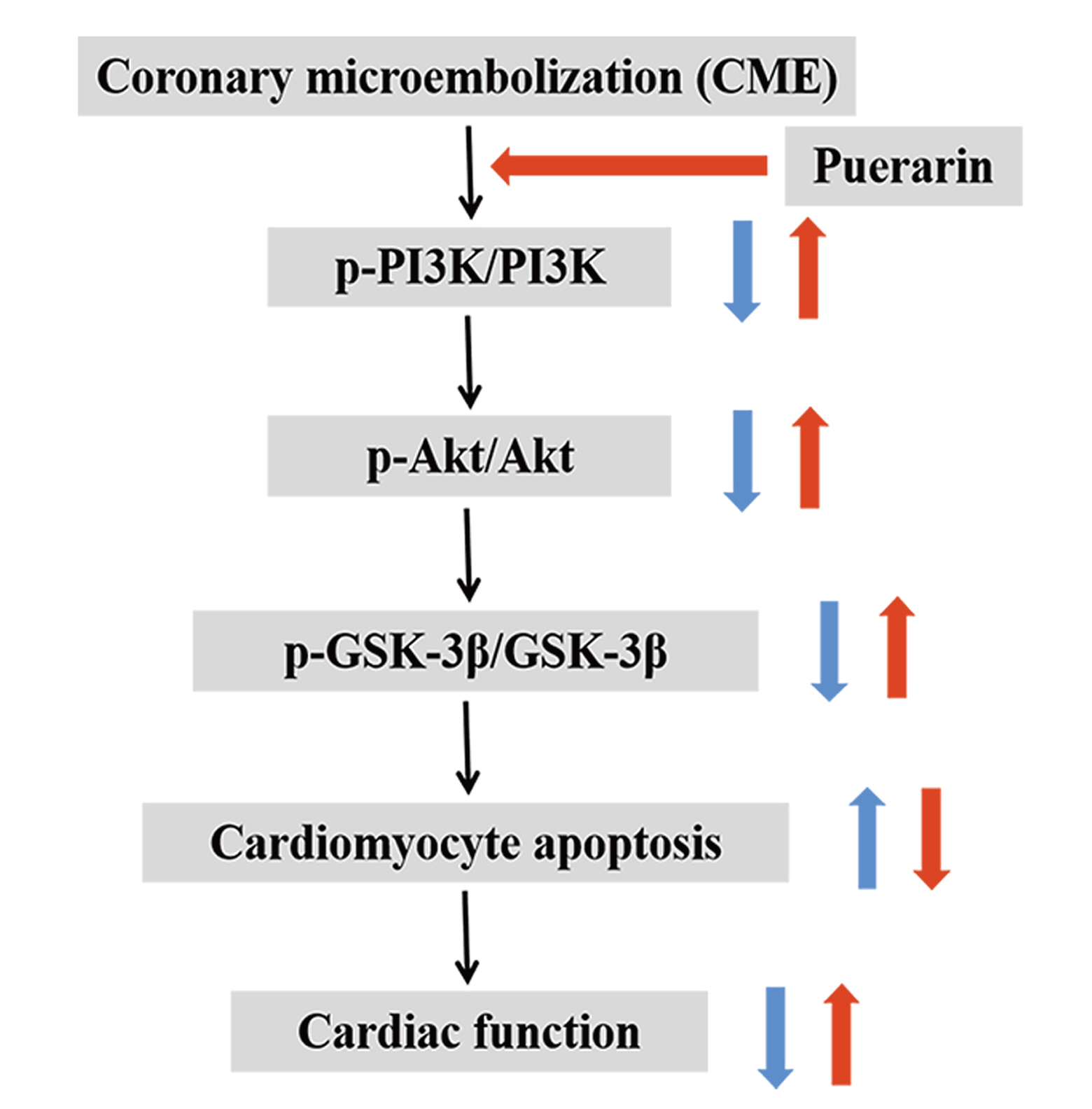
Puerarin pretreatment attenuates cardiomyocyte apoptosis induced by coronary microembolization in rats by activating the PI3K/Akt/GSK-3β signaling pathway
- Affiliations
-
- 1Department of Cardiology, The First Affiliated Hospital of Guangxi Medical University & Guangxi Key Laboratory Base of Precision Medicine in Cardio-cerebrovascular Diseases Control and Prevention & Guangxi Clinical Research Center for Cardio-cerebrovascular Diseases, Nanning, Guangxi 530021, China
- 2Department of Emergency, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi 530021, China
- KMID: 2512958
- DOI: http://doi.org/10.4196/kjpp.2021.25.2.147
Abstract
- Coronary microembolization (CME) is associated with cardiomyocyte apoptosis and cardiac dysfunction. Puerarin confers protection against multiple cardiovascular diseases, but its effects and specific mechanisms on CME are not fully known. Hence, our study investigated whether puerarin pretreatment could alleviate cardiomyocyte apoptosis and improve cardiac function following CME. The molecular mechanism associated was also explored. A total of 48 Sprague-Dawley rats were randomly divided into CME, CME + Puerarin (CME + Pue), sham, and sham + Puerarin (sham + Pue) groups (with 12 rats per group). A CME model was established in CME and CME + Pue groups by injecting 42 μm microspheres into the left ventricle of rats. Rats in the CME + Pue and sham + Pue groups were intraperitoneally injected with puerarin at 120 mg/kg daily for 7 days before operation. Cardiac function, myocardial histopathology, and cardiomyocyte apoptosis index were determined via cardiac ultrasound, hematoxylin-eosin (H&E) and hematoxylin-basic fuchsin-picric acid (HBFP) stainings, and TdT-mediated dUTP nick-end labeling (TUNEL) staining, respectively. Western blotting was used to measure protein expression related to the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/glycogen synthase kinase-3β (GSK-3β) pathway. We found that, puerarin significantly ameliorated cardiac dysfunction after CME, attenuated myocardial infarct size, and reduced myocardial apoptotic index. Besides, puerarin inhibited cardiomyocyte apoptosis, as revealed by decreased Bax and cleaved caspase-3, and up-regulated Bcl-2 and PI3K/Akt/GSK-3β pathway related proteins. Collectively, puerarin can inhibit cardiomyocyte apoptosis and thus attenuate myocardial injury caused by CME. Mechanistically, these effects may be achieved through activation of the PI3K/Akt/GSK-3β pathway.
Figure
Reference
-
1. Heusch G, Skyschally A, Kleinbongard P. 2018; Coronary microembolization and microvascular dysfunction. Int J Cardiol. 258:17–23. DOI: 10.1016/j.ijcard.2018.02.010. PMID: 29429637.
Article2. Scarpone M, Cenko E, Manfrini O. 2018; Coronary no-reflow phenomenon in clinical practice. Curr Pharm Des. 24:2927–2933. DOI: 10.2174/1381612824666180702112536. PMID: 29962336.
Article3. inivasan M Sr, Rihal C, Holmes DR, Prasad A. 2009; Adjunctive thrombectomy and distal protection in primary percutaneous coronary intervention: impact on microvascular perfusion and outcomes. Circulation. 119:1311–1319. DOI: 10.1161/CIRCULATIONAHA.108.831453. PMID: 19273732.4. He WK, Su Q, Liang JB, Wang XT, Sun YH, Li L. 2018; Nicorandil pretreatment inhibits myocardial apoptosis and improves cardiac function after coronary microembolization in rats. J Geriatr Cardiol. 15:591–597. DOI: 10.11909/j.issn.1671-5411.2018.09.002. PMID: 30344543. PMCID: PMC6188981.5. Xue Y, Sun C, Hao Q, Cheng J. 2019; Astaxanthin ameliorates cardiomyocyte apoptosis after coronary microembolization by inhibiting oxidative stress via Nrf2/HO-1 pathway in rats. Naunyn Schmiedebergs Arch Pharmacol. 392:341–348. DOI: 10.1007/s00210-018-1595-0. PMID: 30506291.
Article6. Liu YC, Li L, Su Q, Liu T, Tang ZL. 2015; Trimetazidine pretreatment inhibits myocardial apoptosis and improves cardiac function in a Swine model of coronary microembolization. Cardiology. 130:130–136. DOI: 10.1159/000369246. PMID: 25612843.
Article7. Wang J, Chen H, Su Q, Zhou Y, Liu T, Li L. 2016; The PTEN/Akt signaling pathway mediates myocardial apoptosis in swine after coronary microembolization. J Cardiovasc Pharmacol Ther. 21:471–477. DOI: 10.1177/1074248415624158. PMID: 26846271.
Article8. Su Q, Li L, Zhao J, Sun Y, Yang H. 2017; Effects of nicorandil on PI3K/Akt signaling pathway and its anti-apoptotic mechanisms in coronary microembolization in rats. Oncotarget. 8:99347–99358. DOI: 10.18632/oncotarget.19966. PMID: 29245906. PMCID: PMC5725097.
Article9. Su Q, Lv X, Ye Z. 2019; Ligustrazine attenuates myocardial injury induced by coronary microembolization in rats by activating the PI3K/Akt pathway. Oxid Med Cell Longev. 2019:6791457. DOI: 10.1155/2019/6791457. PMID: 31191802. PMCID: PMC6525935.
Article10. Wei SY, Chen Y, Xu XY. 2014; Progress on the pharmacological research of puerarin: a review. Chin J Nat Med. 12:407–414. DOI: 10.1016/S1875-5364(14)60064-9. PMID: 24969520.
Article11. He L, Wang T, Chen BW, Lu FM, Xu J. 2019; Puerarin inhibits apoptosis and inflammation in myocardial cells via PPARα expression in rats with chronic heart failure. Exp Ther Med. 18:3347–3356. DOI: 10.3892/etm.2019.7984. PMID: 31602208. PMCID: PMC6777288.
Article12. Yin MS, Zhang YC, Xu SH, Liu JJ, Sun XH, Liang C, Wang Y, Li J, Wang FW, Wang QL, Mu YL. 2019; Puerarin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of inflammation. J Asian Nat Prod Res. 21:476–493. DOI: 10.1080/10286020.2017.1405941. PMID: 29322879.13. Xu HX, Pan W, Qian JF, Liu F, Dong HQ, Liu QJ. 2019; MicroRNA-21 contributes to the puerarin-induced cardioprotection via suppression of apoptosis and oxidative stress in a cell model of ischemia/reperfusion injury. Mol Med Rep. 20:719–727. DOI: 10.3892/mmr.2019.10266.
Article14. Liu Q, Lu Z, Wang L. 2000; Restrictive effect of puerarin on myocardial infarct area in dogs and its possible mechanism. J Tongji Med Univ. 20:43–45. DOI: 10.1007/BF02887673. PMID: 12845754.15. He H, Shi M, Yang J, Zeng X, Qiao H, Wu L, Li L. 2008; The correlation between angiogenesis and abnormal expression of SERCA2a, phospholamban and the endothelin pathway in heart failure, and improvement by puerarin. Phytother Res. 22:948–956. DOI: 10.1002/ptr.2437. PMID: 18389490.16. Li W, Lu M, Zhang Y, Xia D, Chen Z, Wang L, Yin N, Wang Z. 2017; Puerarin attenuates the daunorubicin-induced apoptosis of H9c2 cells by activating the PI3K/Akt signaling pathway via the inhibition of Ca2+ influx. Int J Mol Med. 40:1889–1894. DOI: 10.3892/ijmm.2017.3186. PMID: 29039532.17. Liang F, Xie S. 2017; Puerarin prevents tumor necrosis factor-α-induced apoptosis of PC12 cells via activation of the PI3K/Akt signaling pathway. Exp Ther Med. 14:813–818. DOI: 10.3892/etm.2017.4545. PMID: 28673004. PMCID: PMC5488681.
Article18. He W, Su Q, Liang J, Sun Y, Wang X, Li L. 2018; The protective effect of nicorandil on cardiomyocyte apoptosis after coronary microembolization by activating Nrf2/HO-1 signaling pathway in rats. Biochem Biophys Res Commun. 496:1296–1301. DOI: 10.1016/j.bbrc.2018.02.003. PMID: 29412163.
Article19. Su Q, Lv X, Sun Y, Ye Z, Kong B, Qin Z. 2018; Role of TLR4/MyD88/NF-κB signaling pathway in coronary microembolization-induced myocardial injury prevented and treated with nicorandil. Biomed Pharmacother. 106:776–784. DOI: 10.1016/j.biopha.2018.07.014. PMID: 29990871.
Article20. Su Q, Li L, Liu T, Wang J, Zhou Y, Liu Y. 2015; Effects of atorvastatin on PDCD4/NF-κB/TNF-α signaling pathway during coronary microembolization of miniature pigs. Exp Mol Pathol. 99:564–569. DOI: 10.1016/j.yexmp.2015.08.022. PMID: 26341137.
Article21. Mao Q, Liang X, Wu Y, Lu Y. 2019; Resveratrol attenuates cardiomyocyte apoptosis in rats induced by coronary microembolization through SIRT1-mediated deacetylation of p53. J Cardiovasc Pharmacol Ther. 24:551–558. DOI: 10.1177/1074248419845916. PMID: 31046448.
Article22. Skyschally A, Walter B, Heusch G. 2013; Coronary microembolization during early reperfusion: infarct extension, but protection by ischaemic postconditioning. Eur Heart J. 34:3314–3321. DOI: 10.1093/eurheartj/ehs434. PMID: 23242190.
Article23. Dörge H, Neumann T, Behrends M, Skyschally A, Schulz R, Kasper C, Erbel R, Heusch G. 2000; Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol. 279:H2587–H2592. DOI: 10.1152/ajpheart.2000.279.6.H2587. PMID: 11087208.
Article24. Skyschally A, Haude M, Dörge H, Thielmann M, Duschin A, van de Sand A, Konietzka I, Büchert A, Aker S, Massoudy P, Schulz R, Erbel R, Heusch G. 2004; Glucocorticoid treatment prevents progressive myocardial dysfunction resulting from experimental coronary microembolization. Circulation. 109:2337–2342. DOI: 10.1161/01.CIR.0000127961.66744.F4. PMID: 15117838.
Article25. Liang J, Li L, Sun Y, He W, Wang X, Su Q. 2017; The protective effect of activating Nrf2/HO-1 signaling pathway on cardiomyocyte apoptosis after coronary microembolization in rats. BMC Cardiovasc Disord. 17:272. DOI: 10.1186/s12872-017-0704-1. PMID: 29065851. PMCID: PMC5655953.
Article26. Wang XT, Lu YX, Sun YH, He WK, Liang JB, Li L. 2017; TAK-242 protects against apoptosis in coronary microembolization-induced myocardial injury in rats by suppressing TLR4/NF-κB signaling pathway. Cell Physiol Biochem. 41:1675–1683. DOI: 10.1159/000471248. PMID: 28359050.
Article27. Zhao GJ, Hou N, Cai SA, Liu XW, Li AQ, Cheng CF, Huang Y, Li LR, Mai YP, Liu SM, Ou CW, Xiong ZY, Chen XH, Chen MS, Luo CF. 2018; Contributions of Nrf2 to puerarin prevention of cardiac hypertrophy and its metabolic enzymes expression in rats. J Pharmacol Exp Ther. 366:458–469. DOI: 10.1124/jpet.118.248369. PMID: 29945930.
Article28. Shi W, Yuan R, Chen X, Xin Q, Wang Y, Shang X, Cong W, Chen K. 2019; Puerarin reduces blood pressure in spontaneously hypertensive rats by targeting eNOS. Am J Chin Med. 47:19–38. DOI: 10.1142/S0192415X19500022. PMID: 30612457.
Article29. Zhou YX, Zhang H, Peng C. 2014; Puerarin: a review of pharmacological effects. Phytother Res. 28:961–975. DOI: 10.1002/ptr.5083. PMID: 24339367.
Article30. Liu S, Ren HB, Chen XL, Wang F, Wang RS, Zhou B, Wang C, Sun YX, Wang YJ. 2015; Puerarin attenuates severe burn-induced acute myocardial injury in rats. Burns. 41:1748–1757. DOI: 10.1016/j.burns.2015.06.001. PMID: 26514700.
Article31. Guo BQ, Xu JB, Xiao M, Ding M, Duan LJ. 2018; Puerarin reduces ischemia/reperfusion-induced myocardial injury in diabetic rats via upregulation of vascular endothelial growth factor A/angiotensin-1 and suppression of apoptosis. Mol Med Rep. 17:7421–7427. DOI: 10.3892/mmr.2018.8754. PMID: 29568939.
Article32. Han JQ, Yu KY, He M. 2012; [Effects of puerarin on the neurocyte apoptosis and p-Akt (Ser473) expressions in rats with cerebral ischemia/reperfusion injury]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 32:1069–1072. Chinese. PMID: 23173255.33. Wang J, Chen H, Zhou Y, Su Q, Liu T, Wang XT, Li L. 2016; Atorvastatin inhibits myocardial apoptosis in a swine model of coronary microembolization by regulating PTEN/PI3K/Akt signaling pathway. Cell Physiol Biochem. 38:207–219. DOI: 10.1159/000438622. PMID: 26784958.
Article34. Zhu HH, Wang XT, Sun YH, He WK, Liang JB, Mo BH, Li L. 2019; MicroRNA-486-5p targeting PTEN protects against coronary microembolization-induced cardiomyocyte apoptosis in rats by activating the PI3K/AKT pathway. Eur J Pharmacol. 855:244–251. DOI: 10.1016/j.ejphar.2019.03.045. PMID: 31075240.
Article35. Yao Y, Wang Y, Kong L, Chen Y, Yang J. 2019; Osthole decreases tau protein phosphorylation via PI3K/AKT/GSK-3β signaling pathway in Alzheimer's disease. Life Sci. 217:16–24. DOI: 10.1016/j.lfs.2018.11.038. PMID: 30471283.
Article36. Feng MG, Liu CF, Chen L, Feng WB, Liu M, Hai H, Lu JM. 2018; MiR-21 attenuates apoptosis-triggered by amyloid-β via modulating PDCD4/PI3K/AKT/GSK-3β pathway in SH-SY5Y cells. Biomed Pharmacother. 101:1003–1007. DOI: 10.1016/j.biopha.2018.02.043. PMID: 29635890.37. Ghaderi S, Alidadiani N, Dilaver N, Heidari HR, Parvizi R, Rahbarghazi R, Soleimani-Rad J, Baradaran B. 2017; Role of glycogen synthase kinase following myocardial infarction and ischemia-reperfusion. Apoptosis. 22:887–897. DOI: 10.1007/s10495-017-1376-0. PMID: 28421373.
Article38. Miura T, Tanno M. 2010; Mitochondria and GSK-3beta in cardioprotection against ischemia/reperfusion injury. Cardiovasc Drugs Ther. 24:255–263. DOI: 10.1007/s10557-010-6234-z. PMID: 20490903.39. Chen X, Yan X, Guo L. 2018; Inhibitory effect of Patrinia on BRL-3A cell apoptosis through the TLR4/PI3K/AKT/GSK3β and TLR4/P38/JNK signaling pathways. Mol Med Rep. 17:5344–5349. DOI: 10.3892/mmr.2018.8466. PMID: 29363726.
Article40. Wang D, Zhang X, Li D, Hao W, Meng F, Wang B, Han J, Zheng Q. 2017; Kaempferide protects against myocardial ischemia/reperfusion injury through activation of the PI3K/Akt/GSK-3β pathway. Mediators Inflamm. 2017:5278218. DOI: 10.1155/2017/5278218. PMID: 28928604. PMCID: PMC5591971.
Article41. Wu N, Zhang X, Bao Y, Yu H, Jia D, Ma C. 2019; Down-regulation of GAS5 ameliorates myocardial ischaemia/reperfusion injury via the miR-335/ROCK1/AKT/GSK-3β axis. J Cell Mol Med. 23:8420–8431. DOI: 10.1111/jcmm.14724. PMID: 31625671. PMCID: PMC6850918.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Curcumin targets vascular endothelial growth factor viaactivating the PI3K/Akt signaling pathway and improves brainhypoxic-ischemic injury in neonatal rats
- Developmental Exposure to Di-(2-ethylhexyl) Phthalate Induces Cerebellar Granule Cell Apoptosis via the PI3K/AKT Signaling Pathway
- Inhibition of the interaction between Hippo/YAP and Akt signaling with ursolic acid and 3′3-diindolylmethane suppresses esophageal cancer tumorigenesis
- PS-341-Induced Apoptosis is Related to JNK-Dependent Caspase 3 Activation and It is Negatively Regulated by PI3K/Akt-Mediated Inactivation of Glycogen Synthase Kinase-3beta in Lung Cancer Cells
- Inhibition of PI3K/Akt signaling suppresses epithelial-to-mesenchymal transition in hepatocellular carcinoma through the Snail/GSK-3/beta-catenin pathway