J Stroke.
2023 Jan;25(1):92-100. 10.5853/jos.2022.02285.
Cortical Thinning in High-Grade Asymptomatic Carotid Stenosis
- Affiliations
-
- 1Department of Neurology, Columbia University Irving Medical Center, New York, NY, USA
- 2Department of Neurology, University of California Los Angeles, Los Angeles, CA, USA
- 3Department of Radiology, Mayo Clinic, Rochester, MN, USA
- 4Department of Biostatistics, University of Alabama at Birmingham, Birmingham, AL, USA
- 5Department of Neurology, Mayo Clinic, Jacksonville, FL, USA
- 6Department of Surgery, University of Maryland, Baltimore, MD, USA
- 7Department of Radiology, Novant Health Clinical Research, Winston-Salem, NC, USA
- 8Department of Neurologic Surgery, Mayo Clinic, Rochester, MN, USA
- 9Department of Neurology, Kaiser Permanente Los Angeles Medical Center, Los Angeles, CA, USA
- 10Department of Surgery, University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH, USA
- 11Department of Neurology, University of Alabama at Birmingham, Birmingham, AL, USA
- KMID: 2539061
- DOI: http://doi.org/10.5853/jos.2022.02285
Abstract
- Background and Purpose
High-grade carotid artery stenosis may alter hemodynamics in the ipsilateral hemisphere, but consequences of this effect are poorly understood. Cortical thinning is associated with cognitive impairment in dementia, head trauma, demyelination, and stroke. We hypothesized that hemodynamic impairment, as represented by a relative time-to-peak (TTP) delay on MRI in the hemisphere ipsilateral to the stenosis, would be associated with relative cortical thinning in that hemisphere.
Methods
We used baseline MRI data from the NINDS-funded Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis–Hemodynamics (CREST-H) study. Dynamic contrast susceptibility MR perfusion-weighted images were post-processed with quantitative perfusion maps using deconvolution of tissue and arterial signals. The protocol derived a hemispheric TTP delay, calculated by subtraction of voxel values in the hemisphere ipsilateral minus those contralateral to the stenosis.
Results
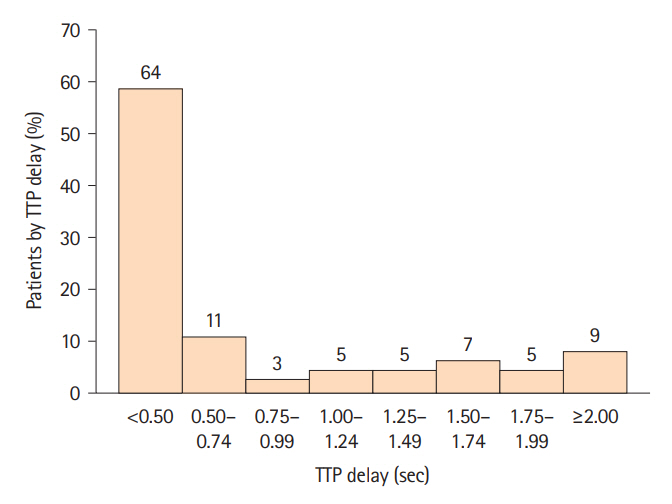
Among 110 consecutive patients enrolled in CREST-H to date, 45 (41%) had TTP delay of at least 0.5 seconds and 9 (8.3%) subjects had TTP delay of at least 2.0 seconds, the maximum delay measured. For every 0.25-second increase in TTP delay above 0.5 seconds, there was a 0.006-mm (6 micron) increase in cortical thickness asymmetry. Across the range of hemodynamic impairment, TTP delay independently predicted relative cortical thinning on the side of stenosis, adjusting for age, sex, hypertension, hemisphere, smoking history, low-density lipoprotein cholesterol, and preexisting infarction (P=0.032).
Conclusions
Our findings suggest that hemodynamic impairment from high-grade asymptomatic carotid stenosis may structurally alter the cortex supplied by the stenotic carotid artery.
Keyword
Figure
Reference
-
References
1. Ko NU, Achrol AS, Martin AJ, Chopra M, Saloner DA, Higashida RT, et al. Magnetic resonance perfusion tracks 133Xe cerebral blood flow changes after carotid stenting. Stroke. 2005; 36:676–678.2. Marshall RS, Asllani I, Pavol MA, Cheung YK, Lazar RM. Altered cerebral hemodyamics and cortical thinning in asymptomatic carotid artery stenosis. PLoS One. 2017; 12:e0189727.3. Teng MM, Cheng HC, Kao YH, Hsu LC, Yeh TC, Hung CS, et al. MR perfusion studies of brain for patients with unilateral carotid stenosis or occlusion: evaluation of maps of “time to peak” and “percentage of baseline at peak”. J Comput Assist Tomogr. 2001; 25:121–125.4. Kluytmans M, van der Grond J, van Everdingen KJ, Klijn CJ, Kappelle LJ, Viergever MA. Cerebral hemodynamics in relation to patterns of collateral flow. Stroke. 1999; 30:1432–1439.5. Reinhard M, Hetzel A, Lauk M, Lücking CH. Dynamic cerebral autoregulation testing as a diagnostic tool in patients with carotid artery stenosis. Neurol Res. 2001; 23:55–63.6. Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000; 283:2122–2127.7. Marshall RS, Pavol MA, Cheung YK, Asllani I, Lazar RM. Cognitive impairment correlates linearly with mean flow velocity by transcranial doppler below a definable threshold. Cerebrovasc Dis Extra. 2020; 10:21–27.8. Balestrini S, Perozzi C, Altamura C, Vernieri F, Luzzi S, Bartolini M, et al. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology. 2013; 80:2145–2150.9. Silvestrini M, Paolino I, Vernieri F, Pedone C, Baruffaldi R, Gobbi B, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology. 2009; 72:1062–1068.10. Cheng CP, Cheng ST, Tam CW, Chan WC, Chu WC, Lam LC. Relationship between cortical thickness and neuropsychological performance in normal older adults and those with mild cognitive impairment. Aging Dis. 2018; 9:1020–1030.11. Kaufmann D, Sollmann N, Kaufmann E, Veggeberg R, Tripodis Y, Wrobel PP, et al. Age at first exposure to tackle football is associated with cortical thickness in former professional American football players. Cereb Cortex. 2021; 31:3426–3434.12. Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010; 21:187–221.13. Seo SW, Ahn J, Yoon U, Im K, Lee JM, Kim ST, et al. Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J Neuroimaging. 2010; 20:37–45.14. Duering M, Righart R, Wollenweber FA, Zietemann V, Gesierich B, Dichgans M. Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology. 2015; 84:1685–1692.15. Lambert C, Sam Narean J, Benjamin P, Zeestraten E, Barrick TR, Markus HS. Characterising the grey matter correlates of leukoaraiosis in cerebral small vessel disease. Neuroimage Clin. 2015; 9:194–205.16. Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, et al. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2011; 54:2659–2671.17. Marshall RS, Lazar RM, Liebeskind DS, Connolly ES, Howard G, Lal BK, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis - Hemodynamics (CREST-H): study design and rationale. Int J Stroke. 2018; 13:985–991.18. Howard VJ, Meschia JF, Lal BK, Turan TN, Roubin GS, Brown RD Jr, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis: protocol of the CREST-2 clinical trials. Int J Stroke. 2017; 12:770–778.19. Watanabe J, Ogata T, Tsuboi Y, Inoue T. Impact of cerebral large-artery disease and blood flow in the posterior cerebral artery territory on cognitive function. J Neurol Sci. 2019; 402:7–11.20. Hochberg AR, Young GS. Cerebral perfusion imaging. Semin Neurol. 2012; 32:454–465.21. Mundiyanapurath S, Ringleb PA, Diatschuk S, Eidel O, Burth S, Floca R, et al. Time-dependent parameter of perfusion imaging as independent predictor of clinical outcome in symptomatic carotid artery stenosis. BMC Neurol. 2016; 16:50.22. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987; 149:351–356.23. Cardinale F, Chinnici G, Bramerio M, Mai R, Sartori I, Cossu M, et al. Validation of FreeSurfer-estimated brain cortical thickness: comparison with histologic measurements. Neuroinformatics. 2014; 12:535–542.24. Frangou S, Modabbernia A, Williams SCR, Papachristou E, Doucet GE, Agartz I, et al. Cortical thickness across the lifespan: data from 17,075 healthy individuals aged 3-90 years. Hum Brain Mapp. 2022; 43:431–451.25. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000; 97:11050–11055.26. Hwang J, Kim CM, Jeon S, Lee JM, Hong YJ, Roh JH, et al. Prediction of Alzheimer’s disease pathophysiology based on cortical thickness patterns. Alzheimers Dement (Amst). 2016; 2:58–67.27. Nickel A, Kessner S, Niebuhr A, Schröder J, Malherbe C, Fischer F, et al. Cortical thickness and cognitive performance in asymptomatic unilateral carotid artery stenosis. BMC Cardiovasc Disord. 2019; 19:154.28. MacDonald ME, Williams RJ, Rajashekar D, Stafford RB, Hanganu A, Sun H, et al. Age-related differences in cerebral blood flow and cortical thickness with an application to age prediction. Neurobiol Aging. 2020; 95:131–142.29. Hachinski V. World stroke day 2008: “little strokes, big trouble”. Stroke. 2008; 39:2407–2420.30. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42:2672–2713.31. Tatemichi TK, Desmond DW, Prohovnik I, Eidelberg D. Dementia associated with bilateral carotid occlusions: neuropsychological and haemodynamic course after extracranial to intracranial bypass surgery. J Neurol Neurosurg Psychiatry. 1995; 58:633–636.32. Marshall RS, Festa JR, Cheung YK, Chen R, Pavol MA, Derdeyn CP, et al. Cerebral hemodynamics and cognitive impairment: baseline data from the RECON trial. Neurology. 2012; 78:250–255.33. Marshall RS, Rundek T, Sproule DM, Fitzsimmons BF, Schwartz S, Lazar RM. Monitoring of cerebral vasodilatory capacity with transcranial Doppler carbon dioxide inhalation in patients with severe carotid artery disease. Stroke. 2003; 34:945–949.34. Huang P, He XY, Xu M. Effects of carotid artery stent and carotid endarterectomy on cognitive function in patients with carotid stenosis. Biomed Res Int. 2020; 2020:6634537.35. Lattanzi S, Carbonari L, Pagliariccio G, Bartolini M, Cagnetti C, Viticchi G, et al. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology. 2018; 90:e307–e315.36. Turowicz A, Czapiga A, Malinowski M, Majcherek J, Litarski A, Janczak D. Carotid revascularization improves cognition in patients with asymptomatic carotid artery stenosis and cognitive decline. Greater improvement in younger patients with more disordered neuropsychological performance. J Stroke Cerebrovasc Dis. 2021; 30:105608.37. Lazar RM, Wadley VG, Myers T, Jones MR, Heck DV, Clark WM, et al. Baseline cognitive impairment in patients with asymptomatic carotid stenosis in the CREST-2 trial. Stroke. 2021; 52:3855–3863.38. Hughes JL, Beech JS, Jones PS, Wang D, Menon DK, Aigbirhio FI, et al. Early-stage 11C-flumazenil PET predicts day-14 selective neuronal loss in a rodent model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2020; 40:1997–2009.39. Karl JM, Alaverdashvili M, Cross AR, Whishaw IQ. Thinning, movement, and volume loss of residual cortical tissue occurs after stroke in the adult rat as identified by histological and magnetic resonance imaging analysis. Neuroscience. 2010; 170:123–137.40. Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004; 14:721–730.41. Kraushar D, Molad J, Hallevi H, Bornstein NM, Ben-Assayag E, Auriel E. Cerebral microinfarcts disruption of remote cortical thickness. J Neurol Sci. 2021; 420:117170.42. Singer OC, de Rochemont Rdu M, Foerch C, Stengel A, Lanfermann H, Sitzer M, et al. Relation between relative cerebral blood flow, relative cerebral blood volume, and mean transit time in patients with acute ischemic stroke determined by perfusion-weighted MRI. J Cereb Blood Flow Metab. 2003; 23:605–611.43. Sobesky J, Zaro Weber O, Lehnhardt FG, Hesselmann V, Thiel A, Dohmen C, et al. Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke. 2004; 35:2843–2847.44. Cheng XQ, Tian JM, Zuo CJ, Liu J, Zhang Q, Lu GM. Quantitative perfusion computed tomography measurements of cerebral hemodynamics: correlation with digital subtraction angiography identified primary and secondary cerebral collaterals in internal carotid artery occlusive disease. Eur J Radiol. 2012; 81:1224–1230.45. Khan AA, Patel J, Desikan S, Chrencik M, Martinez-Delcid J, Caraballo B. Asymptomatic carotid artery stenosis is associated with cerebral hypoperfusion. J Vasc Surg. 2021; 73:1611–1621.e2.46. Lu J, Li KC, Hua Y. Primary study on imaging in transient ischemic attacks. Chin Med J (Engl). 2005; 118:1812–1816.47. Chang CH, Chang TY, Chang YJ, Huang KL, Chin SC, Ryu SJ, et al. The role of perfusion computed tomography in the prediction of cerebral hyperperfusion syndrome. PLoS One. 2011; 6:e19886.48. Muller M, van der Graaf Y, Algra A, Hendrikse J, Mali WP, Geerlings MI; SMART Study Group. Carotid atherosclerosis and progression of brain atrophy: the SMART-MR study. Ann Neurol. 2011; 70:237–244.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Carotid Artery Stenting for Asymptomatic Carotid Stenosis: What We Need to Know for Treatment Decision
- Current Indications of Surgery and Endovascular Treatment in Ischemic Stroke
- Recurrent Carotid Artery Stenosis
- Utility of Acetazolamide-challenged CT Perfusion in Patients with High-grade Carotid Stenosis
- Coexistence of Internal Carotid Artery Stenosis in Patients with Abdominal Aortic Aneurysm