Intest Res.
2023 Jan;21(1):126-136. 10.5217/ir.2021.00166.
Characteristics and usefulness of transabdominal ultrasonography in immune-mediated colitis
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Hokkaido University Hospital, Sapporo, Japan
- 2Division of Laboratory and Transfusion Medicine, Hokkaido University Hospital, Sapporo, Japan
- 3Diagnostic Center for Sonography, Hokkaido University Hospital, Sapporo, Japan
- 4Clinical Research and Medical Innovation Center, Hokkaido University Hospital, Sapporo, Japan
- 5Depatment of Cancer Chemotherapy, Hokkaido University Hospital Cancer Center, Sapporo, Japan
- KMID: 2539011
- DOI: http://doi.org/10.5217/ir.2021.00166
Abstract
- Background/Aims
The usefulness of ultrasonography (US) in diseases of the gastrointestinal tract has been reported recently. This prospective study aimed to determine the features of US findings in immune-mediated colitis (IMC), an adverse event induced by immune checkpoint inhibitor, and examine the correlation between US findings, colonoscopy (CS) findings, and severity of colitis.
Methods
We studied patients examined using CS and US upon suspicion of IMC in Hokkaido University Hospital between April 2018 and February 2021. Endoscopic findings of IMC were assessed using the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). The severity of US findings in IMC was evaluated using US grade, which is the ultrasonographic grading scale in ulcerative colitis. Bowel wall thickness and the intensity of the color Doppler signal were also analyzed. Severity of colitis was evaluated using Common Terminology Criteria for Adverse Events (CTCAE) grade version 5.
Results
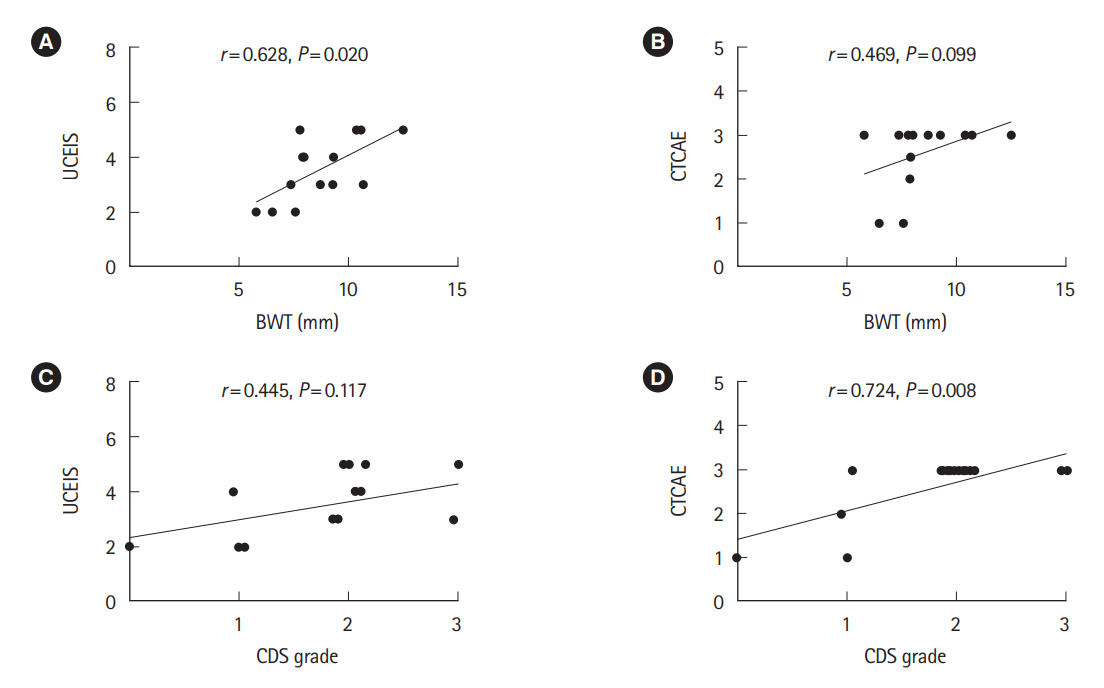
Fourteen patients with IMC were enrolled. The US findings were bowel wall thickening, loss of stratification, ulceration and increased blood flow signal. The US grade was moderately correlated with the UCEIS (r=0.687, p=0.009) and CTCAE grade (r=0.628, p=0.035). Bowel wall thickness and UCEIS (r=0.628, p=0.020), as well as color Doppler signal grade and CTCAE grade (r=0.724, p=0.008), were significantly correlated.
Conclusions
US findings in IMC were mainly similar to those of ulcerative colitis, but there were some findings that were characteristic only of IMC. Significant correlation was found between US findings, CS findings, and severity of colitis. Hence, US could be useful for the evaluation of IMC.
Keyword
Figure
Cited by 2 articles
-
Gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis: a review
Sung Wook Hwang, Min Kyu Kim, Mi-Na Kweon
Intest Res. 2023;21(4):433-442. doi: 10.5217/ir.2023.00019.Endoscopic findings of immune checkpoint inhibitor-related gastrointestinal adverse events
Min Kyu Kim, Sung Wook Hwang
Clin Endosc. 2024;57(6):725-734. doi: 10.5946/ce.2024.003.
Reference
-
1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363:711–723.
Article2. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015; 372:320–330.
Article3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–135.
Article4. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–1639.
Article5. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–1550.
Article6. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015; 33:1430–1437.
Article7. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014; 515:558–562.
Article8. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015; 372:311–319.
Article9. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 372:2509–2520.
Article10. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015; 76–83.
Article11. Nishida T, Iijima H, Adachi S. Immune checkpoint inhibitor-induced diarrhea/colitis: endoscopic and pathologic findings. World J Gastrointest Pathophysiol. 2019; 10:17–28.
Article12. Soularue E, Lepage P, Colombel JF, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut. 2018; 67:2056–2067.
Article13. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015; 16:522–530.
Article14. Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016; 5:e1128611.
Article15. Franklin C, Rooms I, Fiedler M, et al. Cytomegalovirus reactivation in patients with refractory checkpoint inhibitor-induced colitis. Eur J Cancer. 2017; 86:248–256.
Article16. Weber JS, Kähler KC, Hauschild A. Management of immunerelated adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012; 30:2691–2697.
Article17. Yasuda Y, Urata Y, Tohnai R, et al. Immune-related colitis induced by the long-term use of nivolumab in a patient with non-small cell lung cancer. Intern Med. 2018; 57:1269–1272.
Article18. Kubo K, Kato M, Mabe K. Nivolumab-associated colitis mimicking ulcerative colitis. Clin Gastroenterol Hepatol. 2017; 15:A35–A36.
Article19. Yamauchi R, Araki T, Mitsuyama K, et al. The characteristics of nivolumab-induced colitis: an evaluation of three cases and a literature review. BMC Gastroenterol. 2018; 18:135.
Article20. Abu-Sbeih H, Ali FS, Luo W, Qiao W, Raju GS, Wang Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2018; 6:95.
Article21. Geukes Foppen MH, Rozeman EA, van Wilpe S, et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. 2018; 3:e000278.
Article22. Wang Y, Abu-Sbeih H, Mao E, et al. Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis. 2018; 24:1695–1705.
Article23. Wright AP, Piper MS, Bishu S, Stidham RW. Systematic review and case series: flexible sigmoidoscopy identifies most cases of checkpoint inhibitor-induced colitis. Aliment Pharmacol Ther. 2019; 49:1474–1483.
Article24. Haanen JB, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017; 28(Suppl 4):iv119–iv142.
Article25. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018; 36:1714–1768.26. Abu-Sbeih H, Ali FS, Naqash AR, et al. Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J Clin Oncol. 2019; 37:2738–2745.
Article27. Travis SP, Schnell D, Krzeski P, et al. Reliability and initial validation of the Ulcerative Colitis Endoscopic Index of Severity. Gastroenterology. 2013; 145:987–995.
Article28. Vuitton L, Peyrin-Biroulet L, Colombel JF, et al. Defining endoscopic response and remission in ulcerative colitis clinical trials: an international consensus. Aliment Pharmacol Ther. 2017; 45:801–813.
Article29. Cheung VT, Gupta T, Olsson-Brown A, et al. Immune checkpoint inhibitor-related colitis assessment and prognosis: can IBD scoring point the way? Br J Cancer. 2020; 123:207–215.
Article30. Damore LJ 2nd, Rantis PC, Vernava AM 3rd, Longo WE. Colonoscopic perforations: etiology, diagnosis, and management. Dis Colon Rectum. 1996; 39:1308–1314.31. Levin TR, Conell C, Shapiro JA, Chazan SG, Nadel MR, Selby JV. Complications of screening flexible sigmoidoscopy. Gastroenterology. 2002; 123:1786–1792.
Article32. Parente F, Greco S, Molteni M, et al. Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment Pharmacol Ther. 2003; 18:1009–1016.
Article33. Moreno N, Ripollés T, Paredes JM, et al. Usefulness of abdominal ultrasonography in the analysis of endoscopic activity in patients with Crohn’s disease: changes following treatment with immunomodulators and/or anti-TNF antibodies. J Crohns Colitis. 2014; 8:1079–1087.
Article34. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index: National Cooperative Crohn’s Disease Study. Gastroenterology. 1976; 70:439–444.35. Migaleddu V, Scanu AM, Quaia E, et al. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn’s disease. Gastroenterology. 2009; 137:43–52.
Article36. Kinoshita K, Katsurada T, Nishida M, et al. Usefulness of transabdominal ultrasonography for assessing ulcerative colitis: a prospective, multicenter study. J Gastroenterol. 2019; 54:521–529.
Article37. Yamanashi K, Katsurada T, Nishida M, et al. Crohn’s disease activity evaluation by transabdominal ultrasonography: correlation with double-balloon endoscopy. J Ultrasound Med. 2021; 40:2595–2605.
Article38. Nishida M, Shigematsu A, Sato M, et al. Ultrasonographic evaluation of gastrointestinal graft-versus-host disease after hematopoietic stem cell transplantation. Clin Transplant. 2015; 129:697–704.
Article39. Calabrese E, Maaser C, Zorzi F, et al. Bowel ultrasonography in the management of Crohn’s disease: a review with recommendations of an International Panel of Experts. Inflamm Bowel Dis. 2016; 22:1168–1183.
Article40. Migaleddu V, Quaia E, Scano D, Virgilio G. Inflammatory activity in Crohn disease: ultrasound findings. Abdom Imaging. 2008; 33:589–597.
Article41. Fraquelli M, Sarno A, Girelli C, et al. Reproducibility of bowel ultrasonography in the evaluation of Crohn’s disease. Dig Liver Dis. 2008; 40:860–866.
Article42. Parente F, Maconi G, Bollani S, et al. Bowel ultrasound in assessment of Crohn’s disease and detection of related small bowel strictures: a prospective comparative study versus X ray and intraoperative findings. Gut. 2002; 50:490–495.
Article43. Parente F, Greco S, Molteni M, et al. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn’s disease: a prospective comparison with conventional ultrasound, X ray studies, and ileocolonoscopy. Gut. 2004; 53:1652–1657.
Article44. Dietrich CF. Significance of abdominal ultrasound in inflammatory bowel disease. Dig Dis. 2009; 27:482–493.
Article45. Martínez MJ, Ripollés T, Paredes JM, Blanc E, Martí-Bonmatí L. Assessment of the extension and the inflammatory activity in Crohn’s disease: comparison of ultrasound and MRI. Abdom Imaging. 2009; 34:141–148.
Article46. National Cancer Institute (NCI). NCI Common Terminology Criteria for Adverse Events (CTCAE) v5.0 data files. c2019 [cited July 12 2021]. https://evs.nci.nih.gov/ftp1/CTCAE/About.html.47. Sagami S, Kobayashi T, Miyatani Y, et al. Accuracy of ultrasound for evaluation of colorectal segments in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021; 19:908–921.
Article48. Omotehara S, Nishida M, Nagashima K, et al. Immune checkpoint inhibitor-induced colitis successfully followed up by ultrasonography. SN Compr Clin Med. 2020; 2:215–221.
Article49. Marthey L, Mateus C, Mussini C, et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016; 10:395–401.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune Thrombocytopenic Purpur and Neutropenia Associatedwith Ulcerative Colitis
- Pathophysiology of ulcerative colitis - Relationship with genetics and immunity
- Gastric Subepithelial Tumor Diagnosed by Transabdominal Ultrasonography
- Gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis: a review
- The Usefulness of the Transabdominal Ultrasonography as a Screening Examination in the Evaluation of the Patient with Suspicious Gastric Disease