Kosin Med J.
2022 Dec;37(4):320-341. 10.7180/kmj.22.140.
The effects of rebamipide, sucralfate, and rifaximin against inflammation and apoptosis in radiation-induced murine intestinal injury
- Affiliations
-
- 1Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea
- 2Department of Radiation Oncology, Kosin University College of Medicine, Busan, Korea
- 3Kosin University College of Medicine, Busan, Korea
- KMID: 2538863
- DOI: http://doi.org/10.7180/kmj.22.140
Abstract
- Background
Radiotherapy improves overall survival in patients with abdominopelvic malignancies. However, the toxic effects of radiation restrict the maximum dose that can be given, and there are no well-established preventive or therapeutic strategies. This study was conducted to evaluate whether rebamipide, sucralfate, and rifaximin have a suppressive effect on acute ionizing radiation (IR)-induced inflammation in the intestines of mice.
Methods
Thirty-six ICR mice were divided into a vehicle-treated group with sham irradiation; a vehicle-treated group with irradiation; rebamipide, sucralfate, or rifaximin-treated groups with irradiation; and a rebamipide-treated group with sham irradiation. The expression of proinflammatory, anti-inflammatory, proapoptotic, and antiapoptotic factors was investigated.
Results
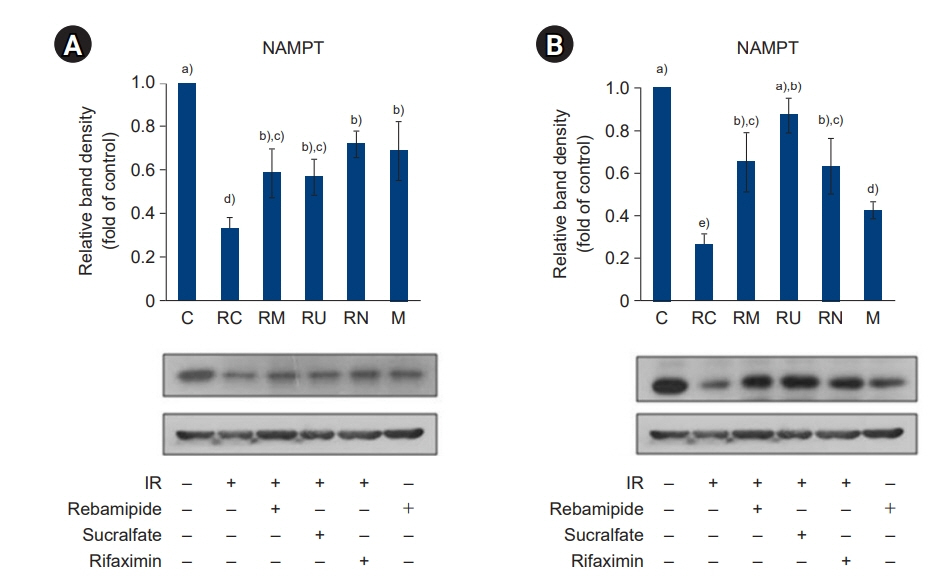
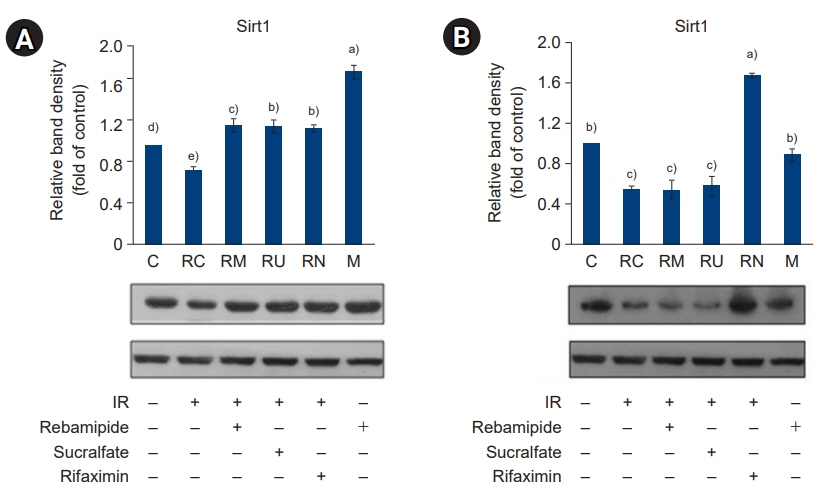
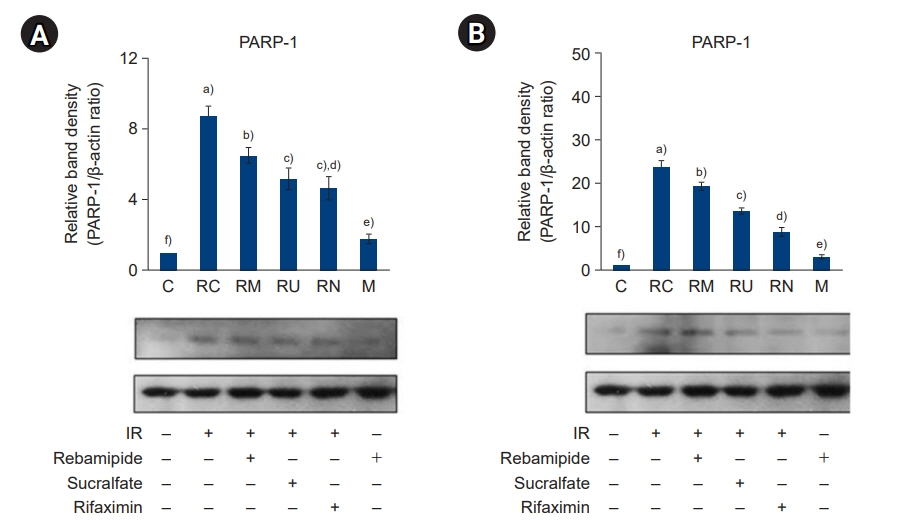
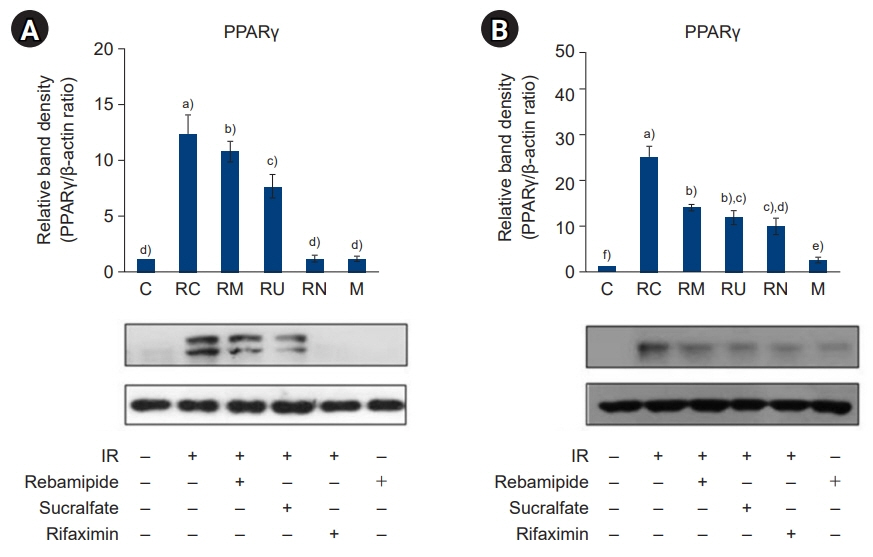
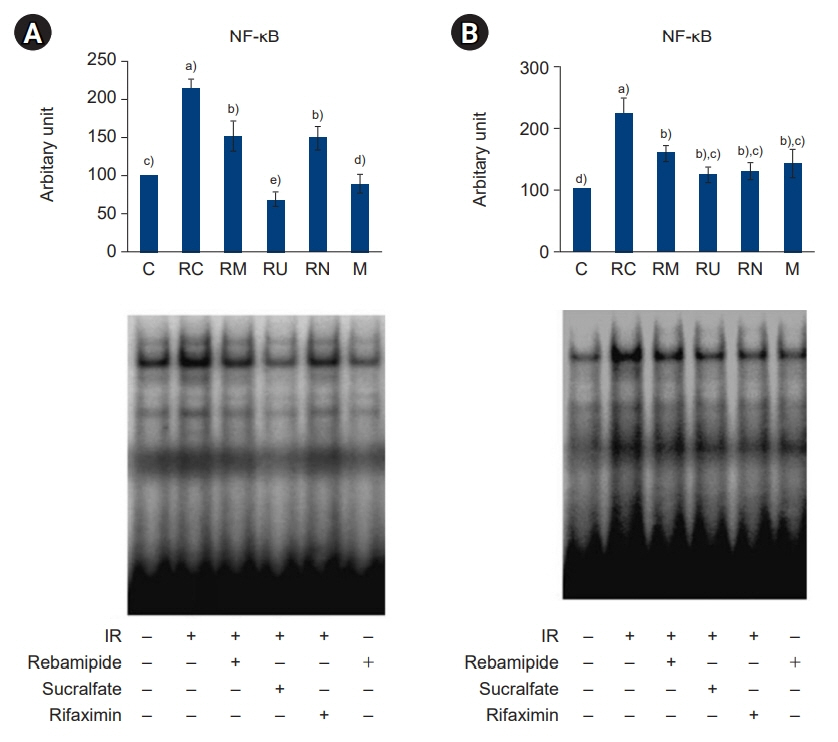
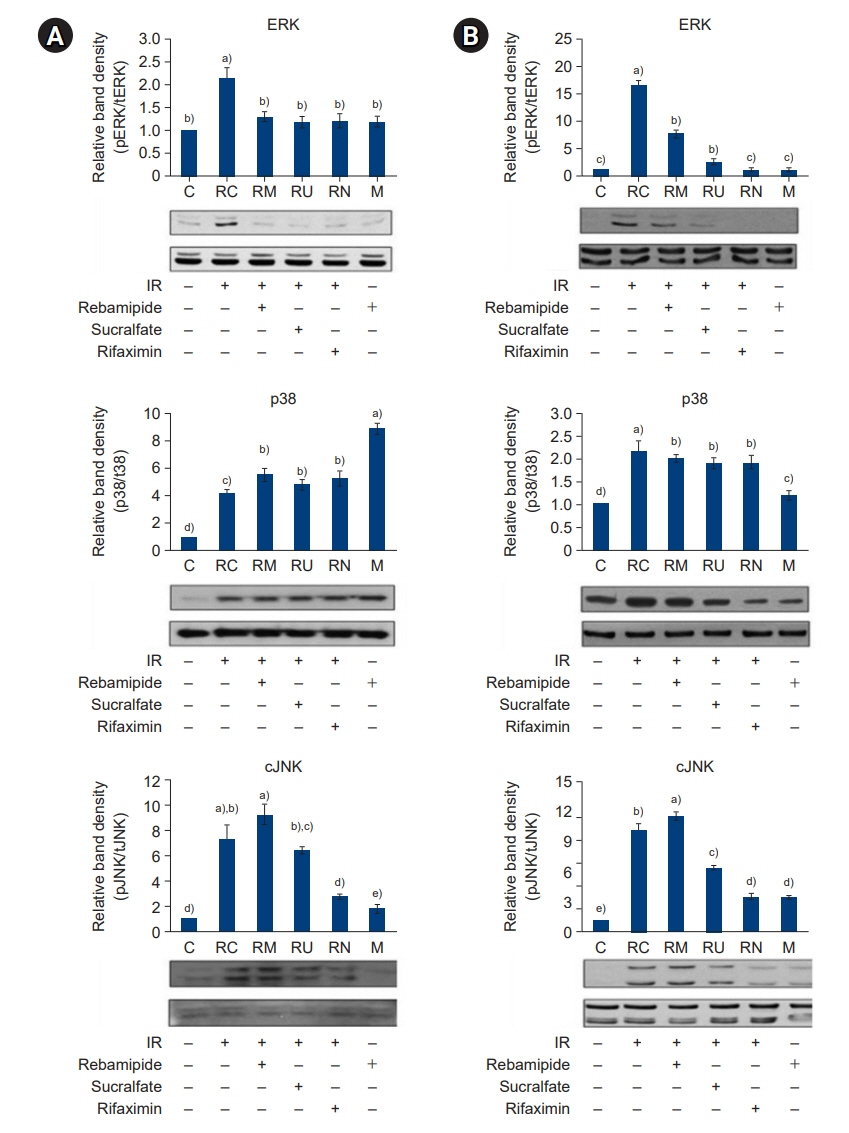
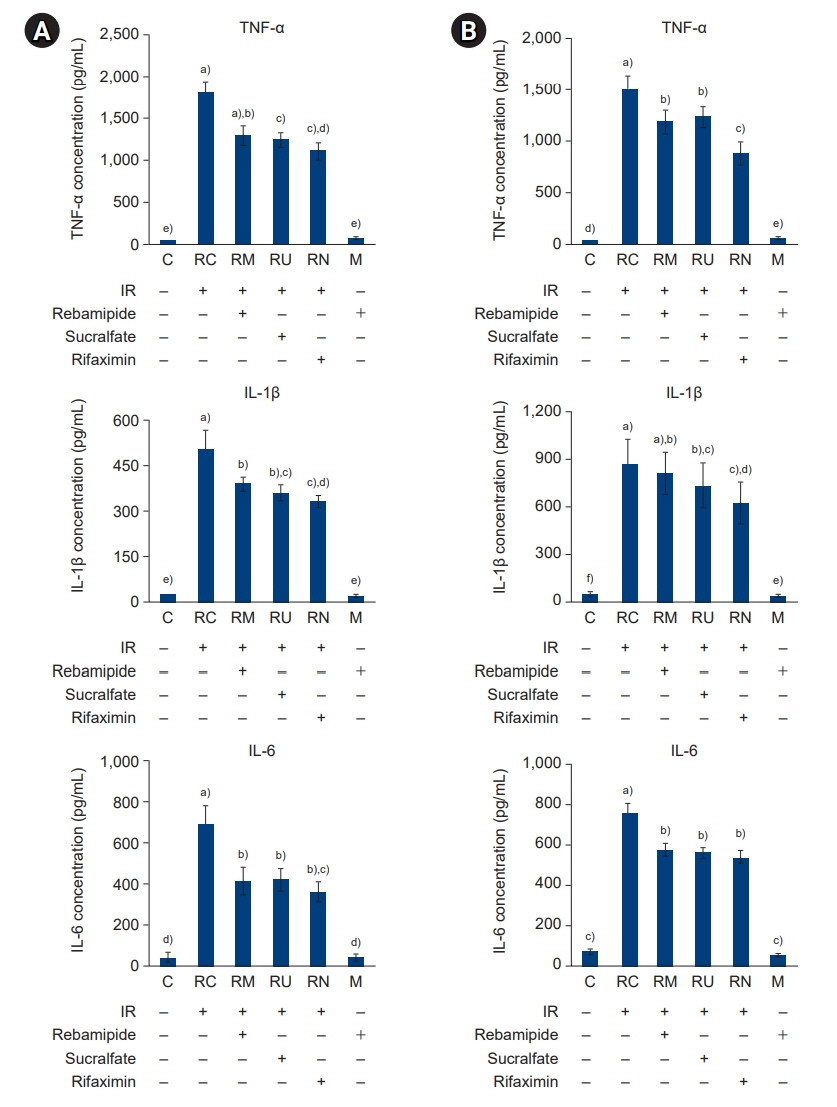
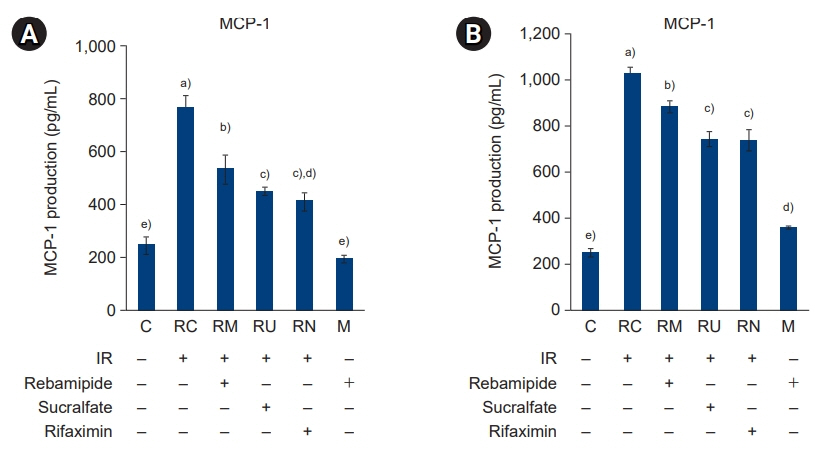
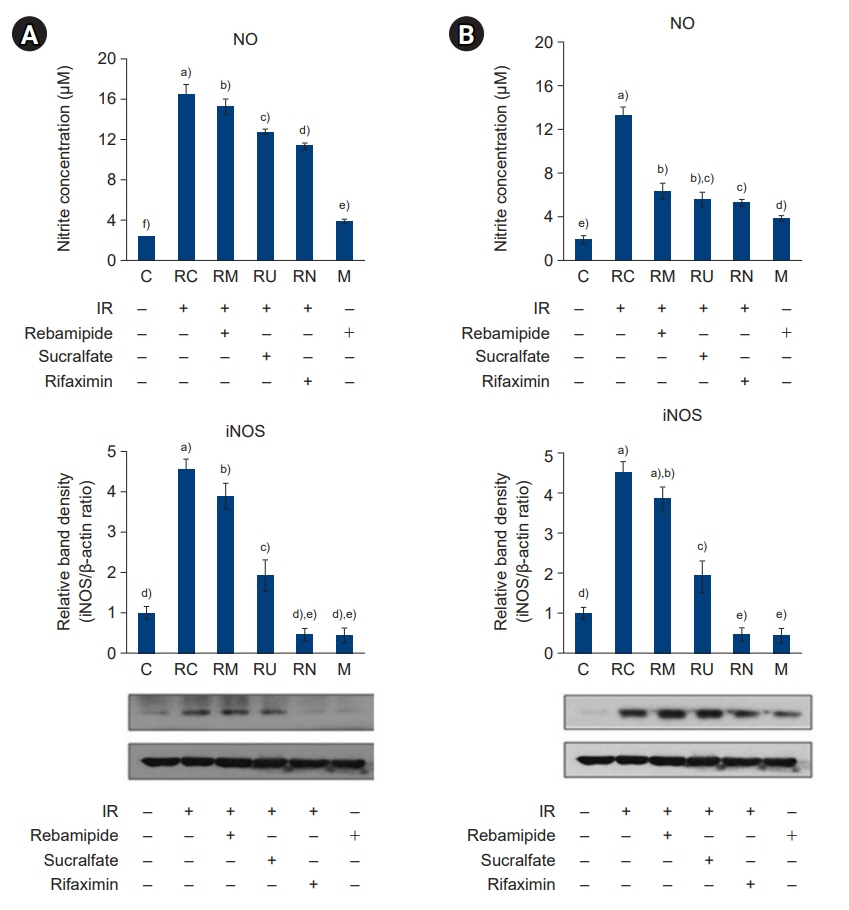
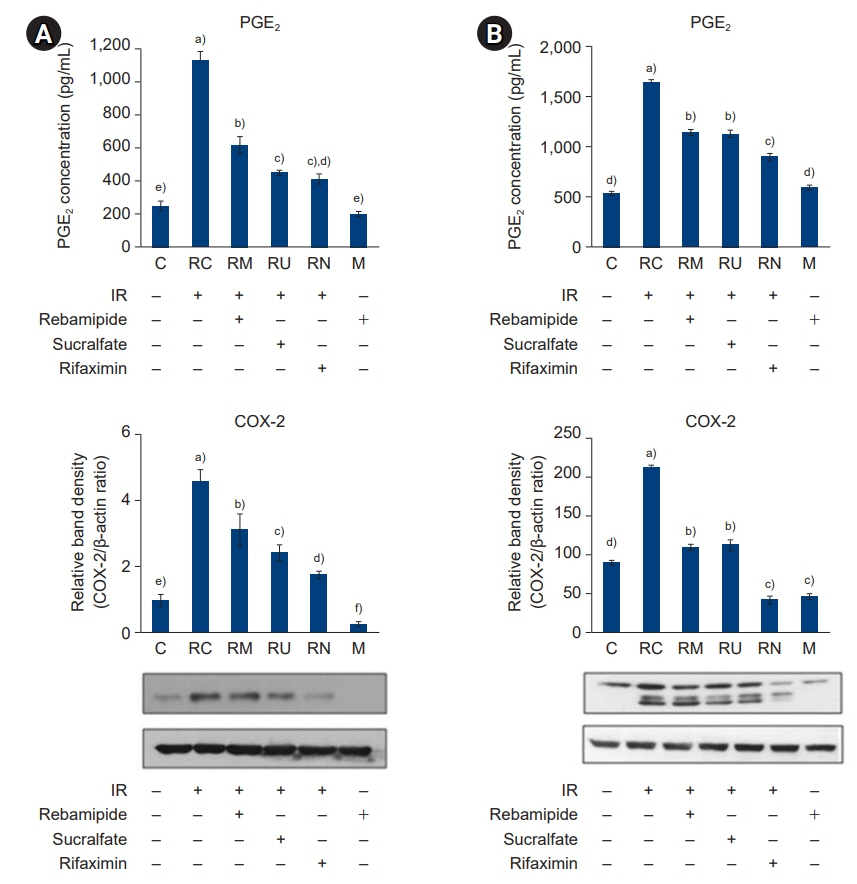
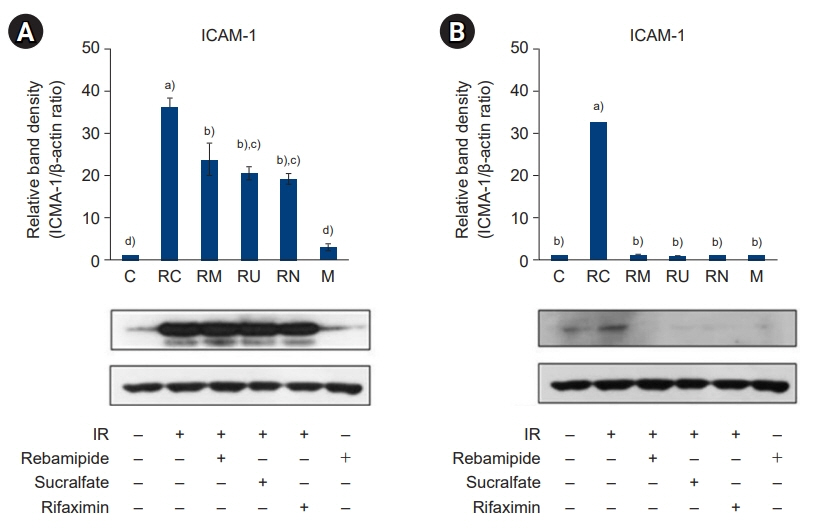
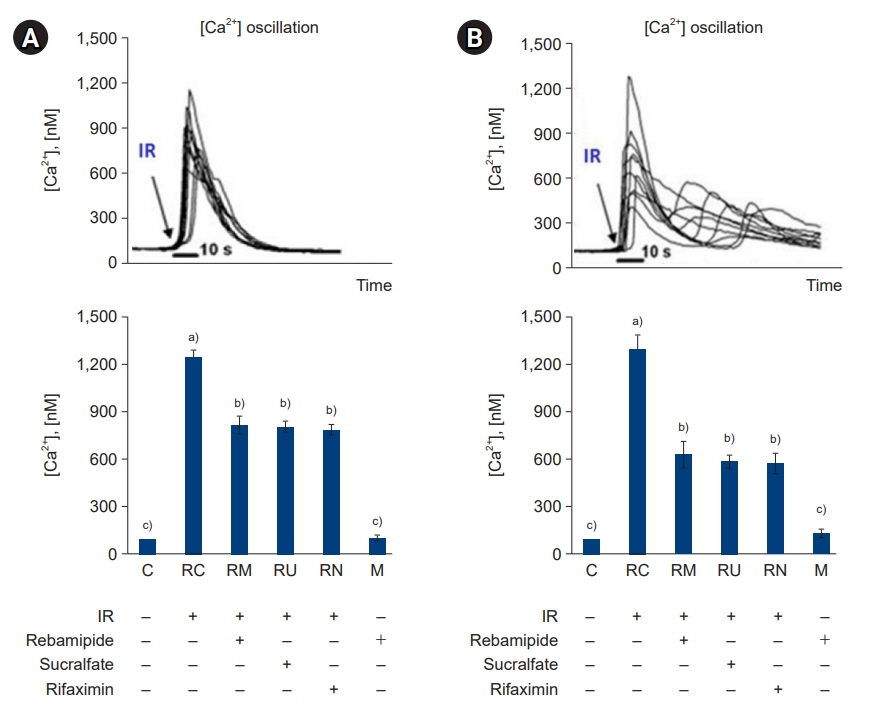
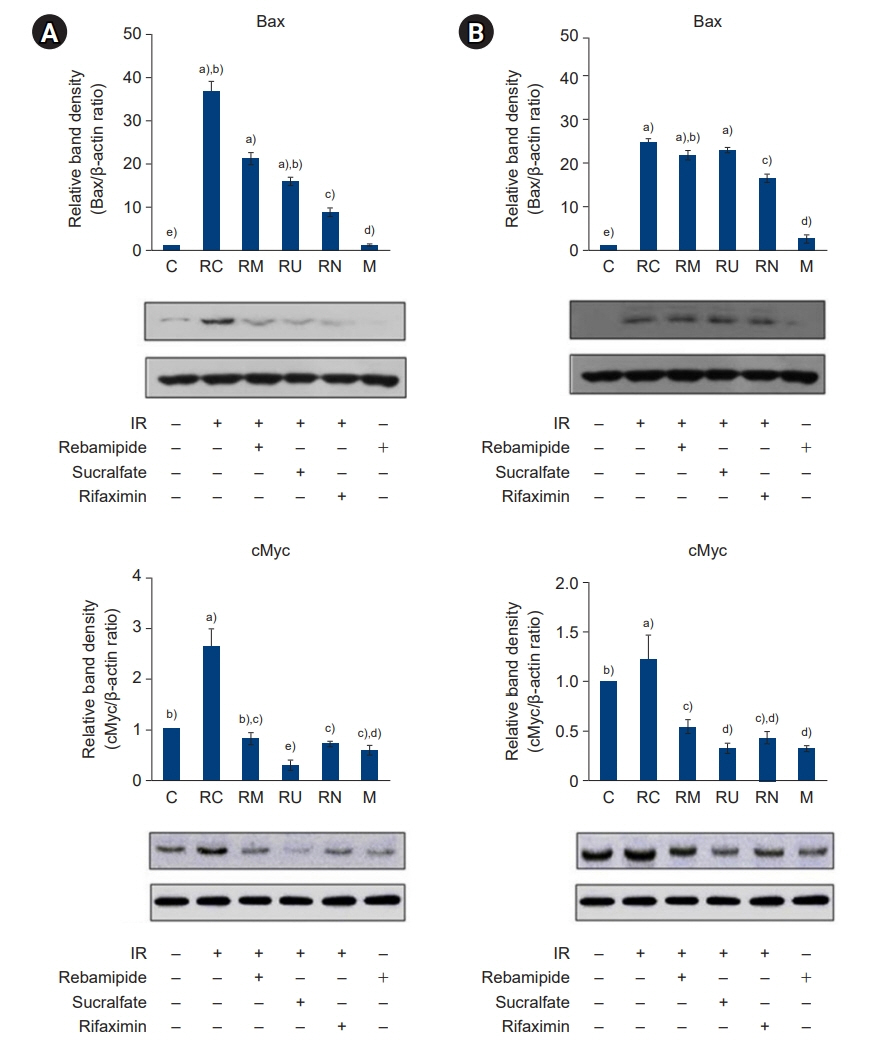
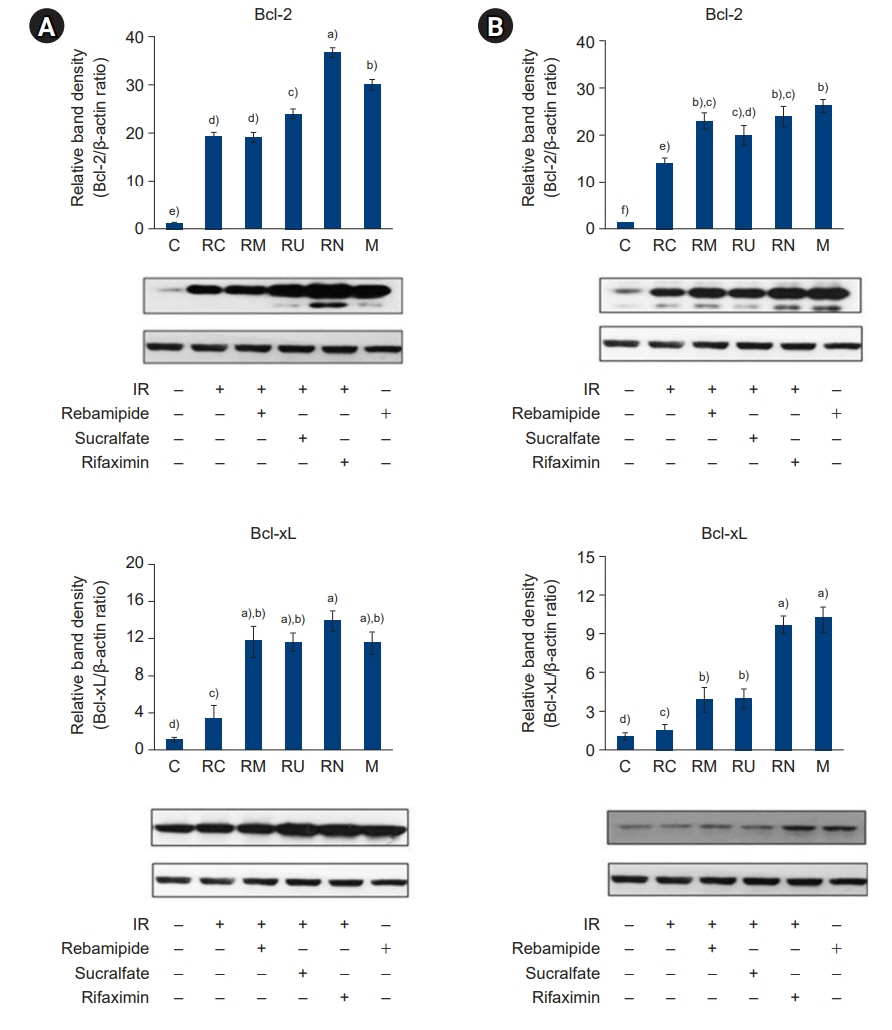
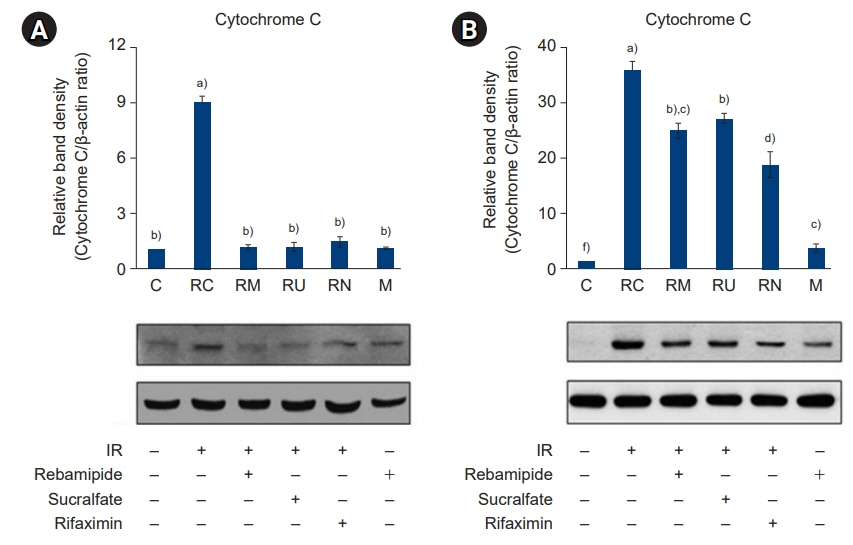
The downregulated expression of nicotinamide phosphoribosyltransferase by IR was attenuated by all drugs (p<0.05). All drugs suppressed the IR-induced activation of NF-κB and phosphorylation of MAPKs (p<0.05) and attenuated the production of TNF-α, IL-1β, and IL-6 in response to IR (p<0.05). The administration of all drugs markedly attenuated IR-induced increases in iNOS, COX-2, and PGE2 (p<0.05), as well as [Ca2+] oscillations that were increased by IR. The expression of proapoptotic genes and antiapoptotic genes was suppressed and induced, respectively, by all drugs. IR treatment increased the release of cytochrome C, which was attenuated by all drugs (p<0.05). All drug treatments resulted in a significant decrease in the expression of caspase-3 and caspase-7 (p<0.05), which were both upregulated following IR treatment.
Conclusions
The administration of rebamipide, sucralfate, or rifaximin prior to radiation therapy may prevent or attenuate acute radiation-induced enterocolitis.
Keyword
Figure
Reference
-
References
1. Koh SB. The ideal strategies of chemotherapy for the treatment of cervical cancer. Kosin Med J. 2018; 33:283–8.
Article2. Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977; 269:518–21.
Article3. Mann WJ. Surgical management of radiation enteropathy. Surg Clin North Am. 1991; 71:977–90.
Article4. Zimmermann FB, Feldmann HJ. Radiation proctitis: clinical and pathological manifestations, therapy and prophylaxis of acute and late injurious effects of radiation on the rectal mucosa. Strahlenther Onkol. 1998; 174 Suppl 3:85–9.5. Naito Y, Yoshikawa T. Rebamipide: a gastrointestinal protective drug with pleiotropic activities. Expert Rev Gastroenterol Hepatol. 2010; 4:261–70.
Article6. Kochhar R, Sriram PV, Sharma SC, Goel RC, Patel F. Natural history of late radiation proctosigmoiditis treated with topical sucralfate suspension. Dig Dis Sci. 1999; 44:973–8.7. Livstone EM, Hersh T, Spiro HM, Floch MH. The gastrointestinal microflora of irradiated mice. II. Effect of oral antibiotic administration on the colonic flora and survival of adult mice. Yale J Biol Med. 1970; 42:448–54.8. Ojetti V, Lauritano EC, Barbaro F, Migneco A, Ainora ME, Fontana L, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol. 2009; 5:675–82.
Article9. Kang G, Kim SE. How to write an original article in medicine and medical science. Kosin Med J. 2022; 37:96–101.
Article10. Kim DJ, Kil SY, Son J, Lee HS. How to conduct well-designed clinical research. Kosin Med J. 2022; 37:187–91.
Article11. Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983; 11:1475–89.
Article12. D’Amours D, Desnoyers S, D’Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999; 342(Pt 2):249–68.
Article13. Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008; 83:804–16.
Article14. Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, Woo CW, et al. Extracellular NAMPT promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem. 2008; 283:34833–43.
Article15. Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007; 404:1–13.
Article16. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004; 23:2369–80.
Article17. Leibiger IB, Berggren PO. Sirt1: a metabolic master switch that modulates lifespan. Nat Med. 2006; 12:34–6.
Article18. Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004; 18:1272–82.
Article19. Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011; 21:146–58.
Article20. Linard C, Marquette C, Clarencon D, Galonnier M, Mathieu J, Pennequin A, et al. Acute ileal inflammatory cytokine response induced by irradiation is modulated by subdiaphragmatic vagotomy. J Neuroimmunol. 2005; 168:83–95.
Article21. Ostrau C, Hulsenbeck J, Herzog M, Schad A, Torzewski M, Lackner KJ, et al. Lovastatin attenuates ionizing radiation-induced normal tissue damage in vivo. Radiother Oncol. 2009; 92:492–9.
Article22. Hong EH, Lee SJ, Kim JS, Lee KH, Um HD, Kim JH, et al. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2010; 285:1283–95.
Article23. Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003; 2:717–26.
Article24. Van Deventer SJ. Tumour necrosis factor and Crohn's disease. Gut. 1997; 40:443–8.
Article25. Sen A, Paine SK, Chowdhury IH, Mukherjee A, Choudhuri S, Saha A, et al. Impact of interleukin-6 promoter polymorphism and serum interleukin-6 level on the acute inflammation and neovascularization stages of patients with Eales’ disease. Mol Vis. 2011; 17:2552–63.26. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009; 29:313–26.27. Barnes PJ, Liew FY. Nitric oxide and asthmatic inflammation. Immunol Today. 1995; 16:128–30.
Article28. Kolli VK, Abraham P, Rabi S. Methotrexate-induced nitrosative stress may play a critical role in small intestinal damage in the rat. Arch Toxicol. 2008; 82:763–70.
Article29. Courtois F, Seidman EG, Delvin E, Asselin C, Bernotti S, Ledoux M, et al. Membrane peroxidation by lipopolysaccharide and iron-ascorbate adversely affects Caco-2 cell function: beneficial role of butyric acid. Am J Clin Nutr. 2003; 77:744–50.
Article30. Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993; 268:9049–54.
Article31. Brzozowski T, Konturek PC, Konturek SJ, Brzozowska I, Pawlik T. Role of prostaglandins in gastroprotection and gastric adaptation. J Physiol Pharmacol. 2005; 56 Suppl 5:33–55.32. Muller-Decker K, Furstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog. 2007; 46:705–10.
Article33. Gretzer B, Ehrlich K, Maricic N, Lambrecht N, Respondek M, Peskar BM. Selective cyclo-oxygenase-2 inhibitors and their influence on the protective effect of a mild irritant in the rat stomach. Br J Pharmacol. 1998; 123:927–35.
Article34. Molla M, Gironella M, Miquel R, Tovar V, Engel P, Biete A, et al. Relative roles of ICAM-1 and VCAM-1 in the pathogenesis of experimental radiation-induced intestinal inflammation. Int J Radiat Oncol Biol Phys. 2003; 57:264–73.
Article35. Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999; 284:339–43.
Article36. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001; 292:727–30.
Article37. Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006; 311:847–51.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Small Dose of Radiation on Induction of Apoptosis in Murine Tumors
- Effect of Sucralfate of the Prophylaxia of Adriamycin Induced Mucositis in the Rat
- Rebamipide Protects TNBS Induced Colonic Damage Through Down-regulation of NF-kappaB Activation and Induction of Heme Oxygenase -1 Expression
- The Effect of Sucralfate on the Reduction of Radiation Esophagitis: Clinical and Laboratory Data
- Rebamipide-induced downregulation of phospholipase D inhibits inflammation and proliferation in gastric cancer cells