Ann Surg Treat Res.
2023 Jan;104(1):1-9. 10.4174/astr.2023.104.1.1.
Prognosis according to the timing of recurrence in breast cancer
- Affiliations
-
- 1Division of Breast Surgery, Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Breast and Endocrine Surgery, Department of Surgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2537621
- DOI: http://doi.org/10.4174/astr.2023.104.1.1
Abstract
- Purpose
Clinically, breast cancer can be divided into 4 subtypes based on the presence of hormone receptors, human epidermal growth factor receptor 2 (HER2), and Ki-67. Because the pattern and time of recurrence vary according to the subtype, we evaluated whether there was a difference in overall survival (OS) among the subtypes according to the time and type of recurrence.
Methods
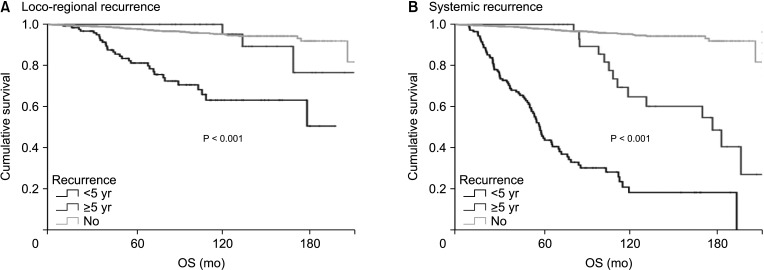
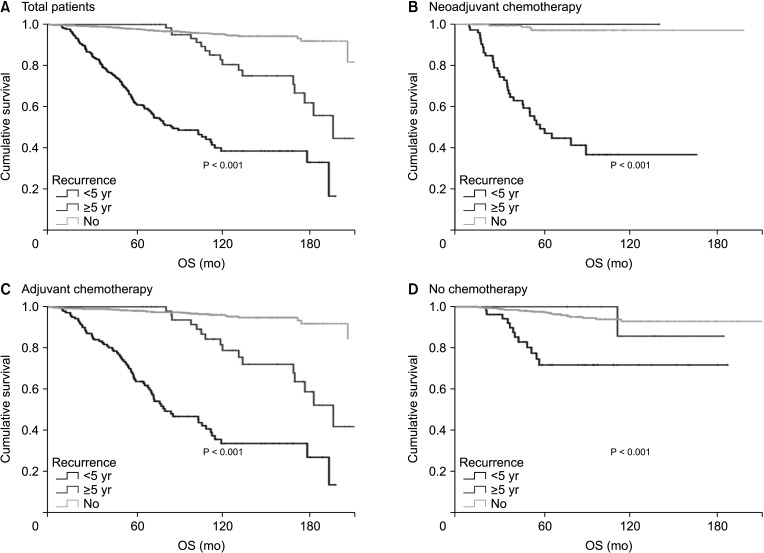
A total of 2,730 patients who underwent breast cancer surgery were analyzed. Early and late recurrence were defined as recurrence within and after 5 years of diagnosis, respectively. Recurrence type was categorized as locoregional recurrence or systemic recurrence.
Results
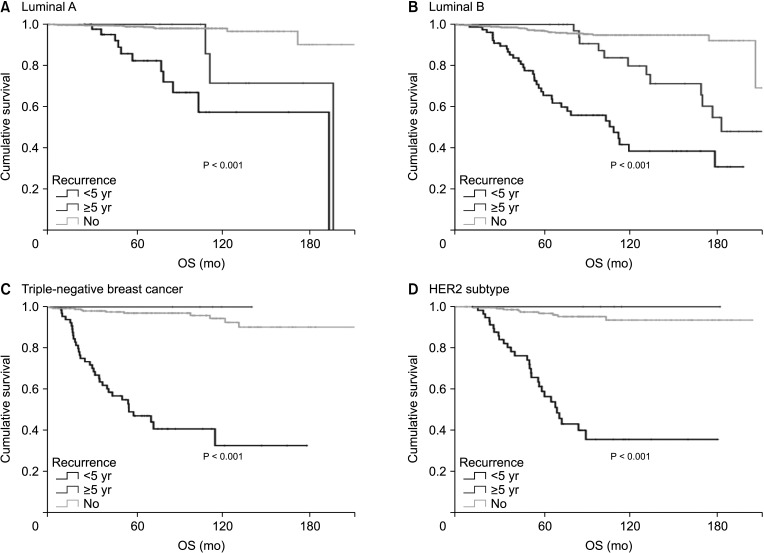
Hormone receptor-positive tumors were significantly more frequent in the late recurrence group than in the early recurrence group (estrogen receptor positive, 47.8% [early] vs. 78.7% [late]). However, there was no difference in the rate of HER2 overexpression (HER2+, 38.1% [early] vs.39.0% [late]). In subgroup analysis, early recurrence was a significant prognostic factor for OS in all subtypes. However, late recurrence was a significant prognostic factor for OS only in the luminal B subtype (hazard ratio of 4.30). In addition, the luminal B type had the highest proportion in late recurrence patients (63.2%).
Conclusion
The luminal B subtype had a high rate of late recurrence, and late recurrence was a poor prognostic factor for OS only in this subgroup. Therefore, further targeted treatments for luminal B breast cancer are needed and patients with this subtype require close long-term surveillance.
Keyword
Figure
Reference
-
1. van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002; 415:530–536. PMID: 11823860.2. Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011; 5:5–23. PMID: 21147047.3. Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013; 31:3083–3090. PMID: 23897954.4. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010; 28:1684–1691. PMID: 20194857.5. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007; 13(15 Pt 1):4429–4434. PMID: 17671126.6. Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009; 27:5700–5706. PMID: 19884543.7. Montagna E, Bagnardi V, Rotmensz N, Viale G, Renne G, Cancello G, et al. Breast cancer subtypes and outcome after local and regional relapse. Ann Oncol. 2012; 23:324–331. PMID: 21525402.8. Goldhirsch A, Gelber RD, Price KN, Castiglione M, Coates AS, Rudenstam CM, et al. Effect of systemic adjuvant treatment on first sites of breast cancer relapse. Lancet. 1994; 343:377–381. PMID: 7905550.9. Tran B, Bedard PL. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011; 13:221. PMID: 22217398.10. De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005; 11:4741–4748. PMID: 16000569.11. de Ronde JJ, Hannemann J, Halfwerk H, Mulder L, Straver ME, Vrancken Peeters MJ, et al. Concordance of clinical and molecular breast cancer subtyping in the context of preoperative chemotherapy response. Breast Cancer Res Treat. 2010; 119:119–126. PMID: 19669409.12. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–750. PMID: 19436038.13. Fry MJ. Phosphoinositide 3-kinase signalling in breast cancer: how big a role might it play? Breast Cancer Res. 2001; 3:304–312. PMID: 11597319.14. Hynes NE, Dey JH. Potential for targeting the fibroblast growth factor receptors in breast cancer. Cancer Res. 2010; 70:5199–5202. PMID: 20570901.15. Creighton CJ, Fu X, Hennessy BT, Casa AJ, Zhang Y, Gonzalez-Angulo AM, et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010; 12:R40. PMID: 20569503.16. Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010; 70:2085–2094. PMID: 20179196.17. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011; 62:233–247. PMID: 20887199.18. Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003; 95:353–361. PMID: 12618500.19. Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997; 277:965–968. PMID: 9252329.20. List HJ, Lauritsen KJ, Reiter R, Powers C, Wellstein A, Riegel AT. Ribozyme targeting demonstrates that the nuclear receptor coactivator AIB1 is a rate-limiting factor for estrogen-dependent growth of human MCF-7 breast cancer cells. J Biol Chem. 2001; 276:23763–23768. PMID: 11328819.21. Yan J, Tsai SY, Tsai MJ. SRC-3/AIB1: transcriptional coactivator in oncogenesis. Acta Pharmacol Sin. 2006; 27:387–394. PMID: 16539836.22. Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004; 96:926–935. PMID: 15199112.23. Alkner S, Jensen MB, Rasmussen BB, Bendahl PO, Fernö M, Rydén L, et al. Prognostic and predictive importance of the estrogen receptor coactivator AIB1 in a randomized trial comparing adjuvant letrozole and tamoxifen therapy in postmenopausal breast cancer: the Danish cohort of BIG 1-98. Breast Cancer Res Treat. 2017; 166:481–490. PMID: 28766132.24. Smith CL, Nawaz Z, O’Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997; 11:657–666. PMID: 9171229.25. Takimoto GS, Graham JD, Jackson TA, Tung L, Powell RL, Horwitz LD, et al. Tamoxifen resistant breast cancer: coregulators determine the direction of transcription by antagonist-occupied steroid receptors. J Steroid Biochem Mol Biol. 1999; 69:45–50. PMID: 10418980.26. Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995; 270:1491–1494. PMID: 7491495.27. Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000; 20:5041–5047. PMID: 10866661.28. Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021; 125:601–610. PMID: 34040177.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Commentary on “Prognosis according to the timing of recurrence in breast cancer” (Ann Surg Treat Res 2023;104:1-9)
- Systemic Treatment After Locoregional Recurrence in Breast Cancer: A Review
- The Optimal Timing of Imaging Examinations in Patients With Newly Diagnosed Breast Cancer in the COVID-19 Pandemic Era
- The Risk Factors Influencing between the Early and Late Recurrence in Systemic Recurrent Breast Cancer
- Treatment Outcomes of Weakly Positive Hormone Receptor Breast Cancer and Triple-Negative Breast Cancer