Korean J Pain.
2023 Jan;36(1):72-83. 10.3344/kjp.22279.
The mechanism of human neural stem cell secretomes improves neuropathic pain and locomotor function in spinal cord injury rat models: through antioxidant, anti-inflammatory, anti-matrix degradation, and neurotrophic activities
- Affiliations
-
- 1Doctoral Program of Medical Science, Faculty of Medicine, Airlangga University, Surabaya, Indonesia
- 2Department of Orthopaedic, Faculty of Medicine, University of Jember, Jember, Indonesia
- 3Department of Orthopaedic, Faculty of Medicine, Airlangga University, Surabaya, Indonesia
- 4Department of Anatomic Pathology, Faculty of Medicine, Airlangga University, Surabaya, Indonesia
- 5Departement of Cardiology, Faculty of Medicine, Airlangga University, Surabaya, Indonesia
- KMID: 2537614
- DOI: http://doi.org/10.3344/kjp.22279
Abstract
- Background
Globally, spinal cord injury (SCI) results in a big burden, including 90% suffering permanent disability, and 60%–69% experiencing neuropathic pain. The main causes are oxidative stress, inflammation, and degeneration. The efficacy of the stem cell secretome is promising, but the role of human neural stem cell (HNSC)-secretome in neuropathic pain is unclear. This study evaluated how the mechanism of HNSC-secretome improves neuropathic pain and locomotor function in SCI rat models through antioxidant, anti-inflammatory, anti-matrix degradation, and neurotrophic activities.
Methods
A proper experimental study investigated 15 Rattus norvegicus divided into normal, control, and treatment groups (30 µL HNSC-secretome, intrathecal in the level of T10, three days post-traumatic SCI). Twentyeight days post-injury, specimens were collected, and matrix metalloproteinase (MMP)-9, F2-Isoprostanes, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, and brain derived neurotrophic factor (BDNF) were analyzed. Locomotor recovery was evaluated via Basso, Beattie, and Bresnahan scores. Neuropathic pain was evaluated using the Rat Grimace Scale.
Results
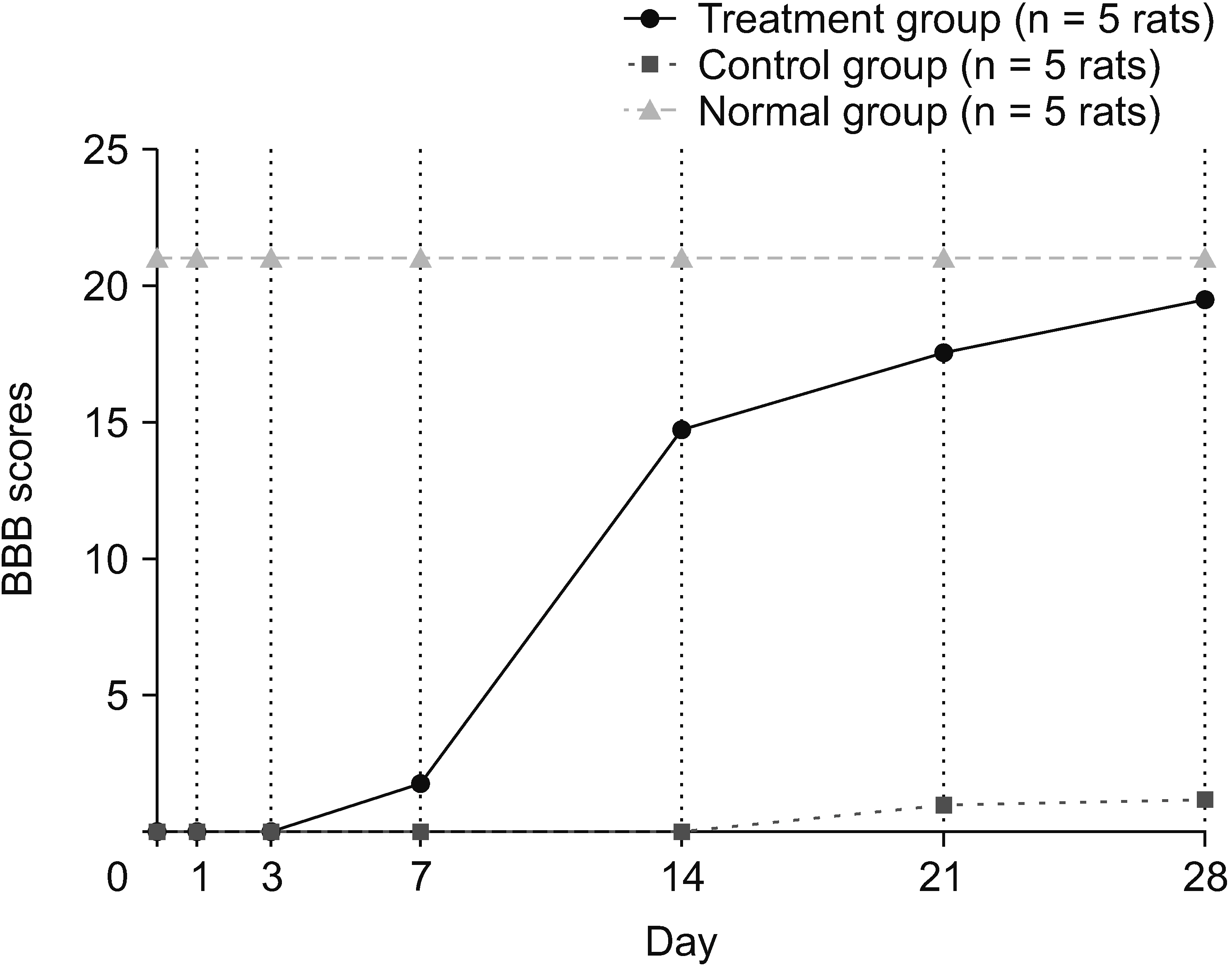
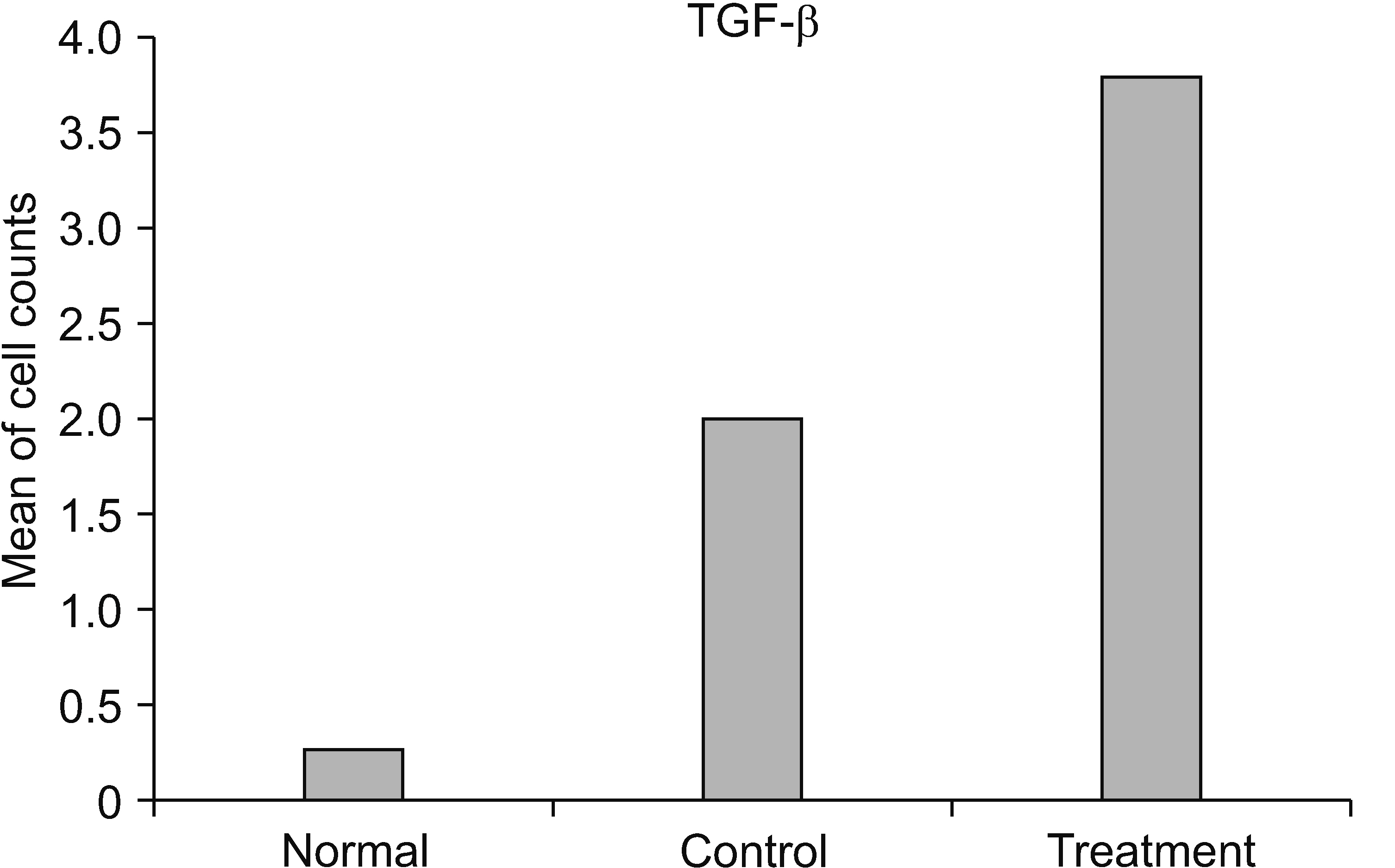
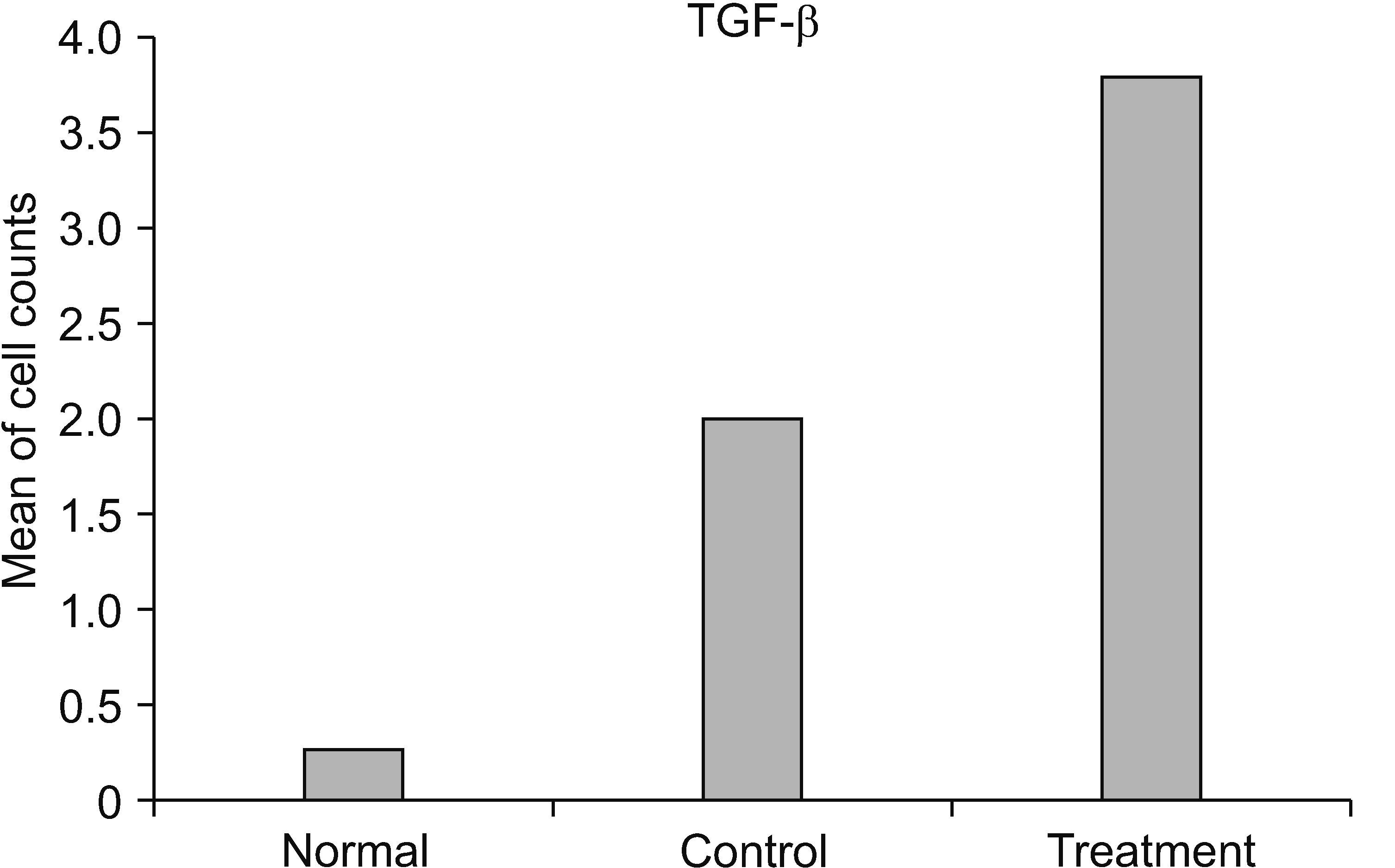
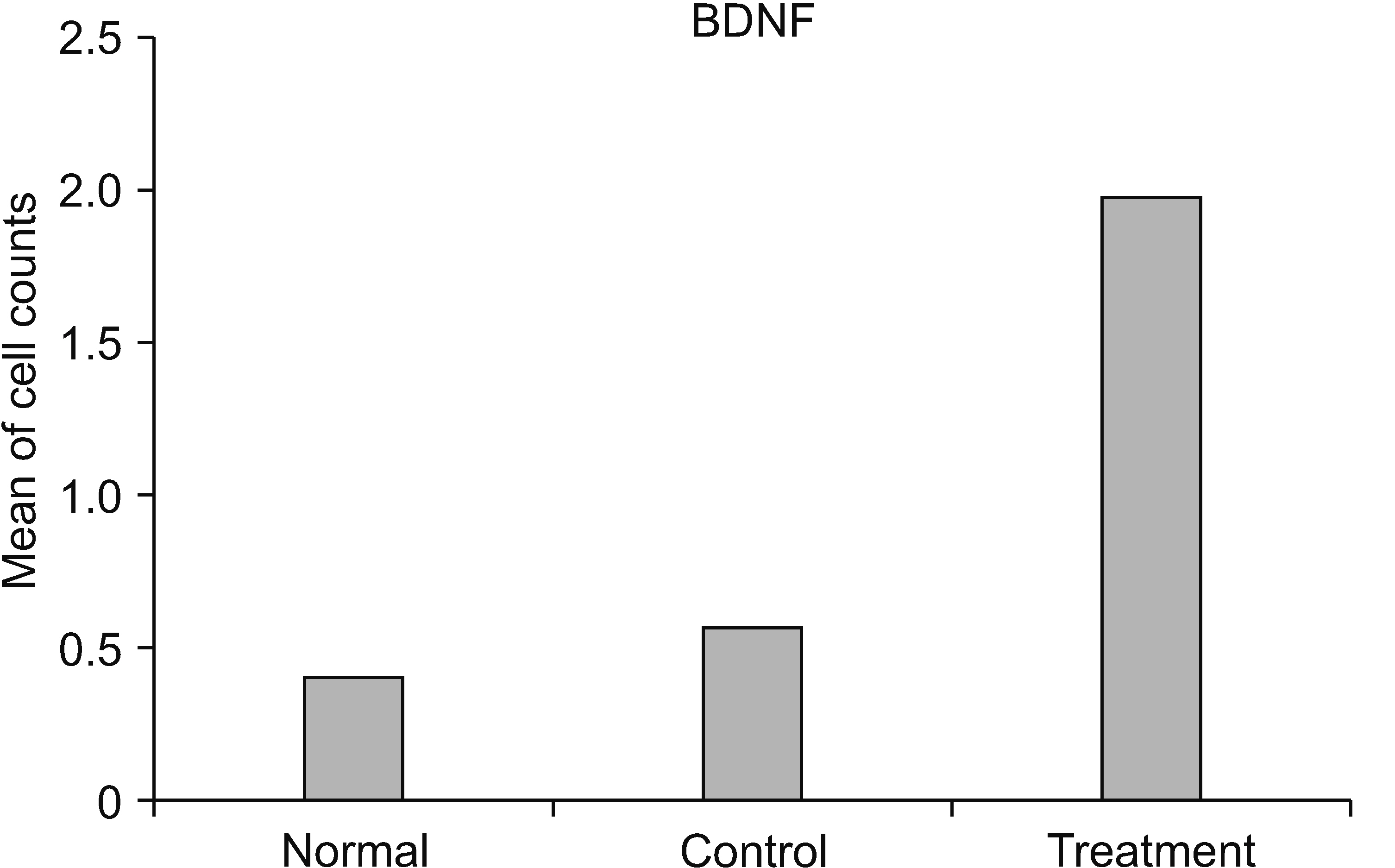
The HNSC-secretome could improve locomotor recovery and neuropathic pain, decrease F2-Isoprostane (antioxidant), decrease MMP-9 and TNF-α (anti-inflammatory), as well as modulate TGF-β and BDNF (neurotrophic factor). Moreover, HNSC-secretomes maintain the extracellular matrix of SCI by reducing the matrix degradation effect of MMP-9 and increasing the collagen formation effect of TGF-β as a resistor of glial scar formation.
Conclusions
The present study demonstrated the mechanism of HNSC-secretome in improving neuropathic pain and locomotor function in SCI through antioxidant, anti-inflammatory, anti-matrix degradation, and neurotrophic activities.
Keyword
Figure
Reference
-
1. Kwon BK, Bloom O, Wanner IB, Curt A, Schwab JM, Fawcett J, et al. 2019; Neurochemical biomarkers in spinal cord injury. Spinal Cord. 57:819–31. DOI: 10.1038/s41393-019-0319-8. PMID: 31273298.2. Miranpuri GS, Nguyen J, Moreno N, Yutuc NA, Kim J, Buttar S, et al. 2019; Folic acid modulates matrix metalloproteinase-9 expression following spinal cord injury. Ann Neurosci. 26:60–5. DOI: 10.5214/ans.0972.7531.260205. PMID: 31975775. PMCID: PMC6894625.3. Hagen EM, Rekand T. 2015; Management of neuropathic pain associated with spinal cord injury. Pain Ther. 4:51–65. DOI: 10.1007/s40122-015-0033-y. PMID: 25744501. PMCID: PMC4470971.4. Davari M, Amani B, Amani B, Khanijahani A, Akbarzadeh A, Shabestan R. 2020; Pregabalin and gabapentin in neuropathic pain management after spinal cord injury: a systematic review and meta-analysis. Korean J Pain. 33:3–12. DOI: 10.3344/kjp.2020.33.1.3. PMID: 31888312. PMCID: PMC6944364.5. Liau LL, Looi QH, Chia WC, Subramaniam T, Ng MH, Law JX. 2020; Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 10:112. DOI: 10.1186/s13578-020-00475-3. PMID: 32983406. PMCID: PMC7510077. PMID: c934b03fdbbd40679f2f624a09545eb3.6. Fakhri S, Sabouri S, Kiani A, Farzaei MH, Rashidi K, Mohammadi-Farani A, et al. 2022; Intrathecal administration of naringenin improves motor dysfunction and neuropathic pain following compression spinal cord injury in rats: relevance to its antioxidant and anti-inflammatory activities. Korean J Pain. 35:291–302. DOI: 10.3344/kjp.2022.35.3.291. PMID: 35768984. PMCID: PMC9251389.7. Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. 2017; Cell transplantation therapy for spinal cord injury. Nat Neurosci. 20:637–47. DOI: 10.1038/nn.4541. PMID: 28440805.8. Pajer K, Bellák T, Nógrádi A. 2021; Stem cell secretome for spinal cord repair: is it more than just a random baseline set of factors? Cells. 10:3214. DOI: 10.3390/cells10113214. PMID: 34831436. PMCID: PMC8625005. PMID: 28646f748099422884a127364368c986.9. Sabelström H, Stenudd M, Réu P, Dias DO, Elfineh M, Zdunek S, et al. 2013; Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 342:637–40. DOI: 10.1126/science.1242576. PMID: 24179227.10. Dilogo IH, Fiolin J. 2019; Role of mesenchymal stem cell-conditioned medium (MSC-CM) in the bone regeneration: a systematic review from 2007-2018. Annu Res Rev Biol. 31:1–16. DOI: 10.9734/arrb/2019/v31i230045.11. Cunningham CJ, Redondo-Castro E, Allan SM. 2018; The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab. 38:1276–92. DOI: 10.1177/0271678X18776802. PMID: 29768965. PMCID: PMC6077926.12. Zhou J, Ni W, Ling Y, Lv X, Niu D, Zeng Y, et al. 2022; Human neural stem cell secretome inhibits lipopolysaccharide-induced neuroinflammation through modulating microglia polarization by activating peroxisome proliferator-activated receptor gamma. Stem Cells Dev. 31:369–82. DOI: 10.1089/scd.2022.0081. PMID: 35481777.13. Mercan A, Uzun ST, Keles S, Hacibeyoglu G, Yilmaz R, Reisli R. 2021; Immunological mechanism of postherpetic neuralgia and effect of pregabalin treatment on the mechanism: a prospective single-arm observational study. Korean J Pain. 34:463–70. DOI: 10.3344/kjp.2021.34.4.463. PMID: 34593664. PMCID: PMC8494950.14. Gao L, Peng Y, Xu W, He P, Li T, Lu X, et al. 2020; Progress in stem cell therapy for spinal cord injury. Stem Cells Int. 2020:2853650. DOI: 10.1155/2020/2853650. PMID: 33204276. PMCID: PMC7661146.15. Haider T, Höftberger R, Rüger B, Mildner M, Blumer R, Mitterbauer A, et al. 2015; The secretome of apoptotic human peripheral blood mononuclear cells attenuates secondary damage following spinal cord injury in rats. Exp Neurol. 267:230–42. DOI: 10.1016/j.expneurol.2015.03.013. PMID: 25797576.16. Oliveri RS, Bello S, Biering-Sørensen F. 2014; Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: systematic review with meta-analyses of rat models. Neurobiol Dis. 62:338–53. DOI: 10.1016/j.nbd.2013.10.014. PMID: 24148857.17. Swenson J, Carpenter JW. Carpenter JW, Marion CJ, editors. 2018. Select topics for the exotic animal veterinarian. Exotic Animal Formulary. 5th ed. Saunders;St. Louis: p. 636–63. DOI: 10.1016/B978-0-323-44450-7.00015-1.18. George RP, Howarth GS, Whittaker AL. 2019; Use of the rat grimace scale to evaluate visceral pain in a model of chemotherapy-induced mucositis. Animals (Basel). 9:678. DOI: 10.3390/ani9090678. PMID: 31547463. PMCID: PMC6769932.19. Klune CB, Larkin AE, Leung VSY, Pang D. 2019; Comparing the Rat Grimace Scale and a composite behaviour score in rats. PLoS One. 14:e0209467. DOI: 10.1371/journal.pone.0209467. PMCID: PMC6544219. PMID: 31150408. PMID: bb1f5f3b33e74560ab6d792241e0a5ff.20. Basso DM, Beattie MS, Bresnahan JC. 1995; A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 12:1–21. DOI: 10.1089/neu.1995.12.1. PMID: 7783230.21. Borhani-Haghighi M, Navid S, Mohamadi Y. 2020; The therapeutic potential of conditioned medium from human breast milk stem cells in treating spinal cord injury. Asian Spine J. 14:131–8. DOI: 10.31616/asj.2019.0026. PMID: 31711062. PMCID: PMC7113460. PMID: b547bda7fca74ab397b25e9ff0b6da56.22. Carter MW, Johnson KM, Lee JY, Hulsebosch CE, Gwak YS. 2016; Comparison of mechanical allodynia and recovery of locomotion and bladder function by different parameters of low thoracic spinal contusion injury in rats. Korean J Pain. 29:86–95. DOI: 10.3344/kjp.2016.29.2.86. PMID: 27103963. PMCID: PMC4837124.23. Leung V, Zhang E, Pang DS. 2016; Real-time application of the Rat Grimace Scale as a welfare refinement in laboratory rats. Sci Rep. 6:31667. DOI: 10.1038/srep31667. PMCID: PMC4987703. PMID: 27530823.24. Wu J, Zhao Z, Zhu X, Renn CL, Dorsey SG, Faden AI. 2016; Cell cycle inhibition limits development and maintenance of neuropathic pain following spinal cord injury. Pain. 157:488–503. DOI: 10.1097/j.pain.0000000000000393. PMID: 26797506. PMCID: PMC4881432.25. Hatch MN, Cushing TR, Carlson GD, Chang EY. 2018; Neuropathic pain and SCI: identification and treatment strategies in the 21st century. J Neurol Sci. 384:75–83. DOI: 10.1016/j.jns.2017.11.018. PMID: 29249383.26. Schneider LE, Henley KY, Turner OA, Pat B, Niedzielko TL, Floyd CL. 2017; Application of the Rat Grimace Scale as a marker of supraspinal pain sensation after cervical spinal cord injury. J Neurotrauma. 34:2982–93. DOI: 10.1089/neu.2016.4665. PMID: 27998207. PMCID: PMC6436031.27. Cunningham CJ, Enrich MV, Pickford MM, MacIntosh-Smith W, Huang W. 2020; The therapeutic potential of the stem cell secretome for spinal cord repair: a systematic review and meta-analysis. OBM Neurobiol. 4:080. DOI: 10.21926/obm.neurobiol.2004080.28. Mietto BS, Mostacada K, Martinez AM. 2015; Neurotrauma and inflammation: CNS and PNS responses. Mediators Inflamm. 2015:251204. DOI: 10.1155/2015/251204. PMID: 25918475. PMCID: PMC4397002.29. Owoyele BV, Bakare AO, Olaseinde OF, Ochu MJ, Yusuff AM, Ekebafe F, et al. 2022; Synergistic interaction between acetaminophen and L-carnosine improved neuropathic pain via NF-κB pathway and antioxidant properties in chronic constriction injury model. Korean J Pain. 35:271–9. DOI: 10.3344/kjp.2022.35.3.271. PMCID: PMC9251391. PMID: 35768982.30. Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. 2012; Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 50:264–74. DOI: 10.1038/sc.2011.111. PMID: 21987065.31. Dos Santos MF, Roxo C, Solá S. 2021; Oxidative-signaling in neural stem cell-mediated plasticity: implications for neurodegenerative diseases. Antioxidants (Basel). 10:1088. DOI: 10.3390/antiox10071088. PMID: 34356321. PMCID: PMC8301193. PMID: db1b2df39a4a439dab7d05ce6359361a.32. Giudicessi JR, Ackerman MJ. 2013; Determinants of incomplete penetrance and variable expressivity in heritable cardiac arrhythmia syndromes. Transl Res. 161:1–14. DOI: 10.1016/j.trsl.2012.08.005. PMID: 22995932. PMCID: PMC3624763.33. Clausen F, Marklund N, Lewén A, Enblad P, Basu S, Hillered L. 2012; Interstitial F(2)-isoprostane 8-iso-PGF(2α) as a biomarker of oxidative stress after severe human traumatic brain injury. J Neurotrauma. 29:766–75. DOI: 10.1089/neu.2011.1754. PMID: 21639729.34. Leung L. 2012; Cellular therapies for treating pain associated with spinal cord injury. J Transl Med. 10:37. DOI: 10.1186/1479-5876-10-37. PMID: 22394650. PMCID: PMC3320547.35. Cheng Z, Zhu W, Cao K, Wu F, Li J, Wang G, et al. 2016; Anti-inflammatory mechanism of neural stem cell transplantation in spinal cord injury. Int J Mol Sci. 17:1380. DOI: 10.3390/ijms17091380. PMID: 27563878. PMCID: PMC5037660.36. Li S, Gu X, Yi S. 2017; The regulatory effects of transforming growth factor-β on nerve regeneration. Cell Transplant. 26:381–94. DOI: 10.3727/096368916X693824. PMID: 27983926. PMCID: PMC5657701.37. Pajer K, Bellák T, Nógrádi A. 2020; The mutual interaction between the host spinal cord and grafted undifferentiated stem cells fosters the production of a lesion-induced secretome. Neural Regen Res. 15:1844–5. DOI: 10.4103/1673-5374.280312. PMID: 32246628. PMCID: PMC7513963. PMID: ea433ad39ec443a49a08bff4503bdb9b.38. Hansen CN, Fisher LC, Deibert RJ, Jakeman LB, Zhang H, Noble-Haeusslein L, et al. 2013; Elevated MMP-9 in the lumbar cord early after thoracic spinal cord injury impedes motor relearning in mice. J Neurosci. 33:13101–11. DOI: 10.1523/JNEUROSCI.1576-13.2013. PMID: 23926264. PMCID: PMC3735886.39. Gomes LR, Terra LF, Wailemann RA, Labriola L, Sogayar MC. 2012; TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer. 12:26. DOI: 10.1186/1471-2407-12-26. PMID: 22260435. PMCID: PMC3277461.40. De Araújo AA, Varela H, Brito GAC, De Medeiros CACX, Araújo LS, Do Nascimento JHO, et al. 2014; Azilsartan increases levels of IL-10, down-regulates MMP-2, MMP-9, RANKL/RANK, cathepsin K and up-regulates OPG in an experimental periodontitis model. PLoS One. 9:e96750. DOI: 10.1371/journal.pone.0096750. PMID: 24819928. PMCID: PMC4018354. PMID: 1a7d800fce3a4ba397ec52b701f69b19.41. Lakhan SE, Avramut M. 2012; Matrix metalloproteinases in neuropathic pain and migraine: friends, enemies, and therapeutic targets. Pain Res Treat. 2012:952906. DOI: 10.1155/2012/952906. PMID: 22970361. PMCID: PMC3434407.42. Anwar MA, Al Shehabi TS, Eid AH. 2016; Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 10:98. DOI: 10.3389/fncel.2016.00098. PMID: 27147970. PMCID: PMC4829593.43. Joko M, Osuka K, Usuda N, Atsuzawa K, Aoyama M, Takayasu M. 2013; Different modifications of phosphorylated Smad3C and Smad3L through TGF-β after spinal cord injury in mice. Neurosci Lett. 549:168–72. DOI: 10.1016/j.neulet.2013.05.042. PMID: 23727390.44. Shahsavari F, Abbasnejad M, Esmaeili-Mahani S, Raoof M. 2022; The ability of orexin-A to modify pain-induced cyclooxygenase-2 and brain-derived neurotrophic factor expression is associated with its ability to inhibit capsaicin-induced pulpal nociception in rats. Korean J Pain. 35:261–70. DOI: 10.3344/kjp.2022.35.3.261. PMID: 35768981. PMCID: PMC9251390.45. Weishaupt N, Li S, Di Pardo A, Sipione S, Fouad K. 2013; Synergistic effects of BDNF and rehabilitative training on recovery after cervical spinal cord injury. Behav Brain Res. 239:31–42. DOI: 10.1016/j.bbr.2012.10.047. PMID: 23131414.46. Leech KA, Hornby TG. 2017; High-intensity locomotor exercise increases brain-derived neurotrophic factor in individuals with incomplete spinal cord injury. J Neurotrauma. 34:1240–8. DOI: 10.1089/neu.2016.4532. PMCID: PMC5359683. PMID: 27526567.47. Boyce VS, Mendell LM. 2014; Neurotrophins and spinal circuit function. Front Neural Circuits. 8:59. DOI: 10.3389/fncir.2014.00059. PMID: 24926235. PMCID: PMC4046666.48. Tashiro S, Shinozaki M, Mukaino M, Renault-Mihara F, Toyama Y, Liu M, et al. 2015; BDNF induced by treadmill training contributes to the suppression of spasticity and allodynia after spinal cord injury via upregulation of KCC2. Neurorehabil Neural Repair. 29:677–89. DOI: 10.1177/1545968314562110. PMID: 25527489.49. Kusiak AN, Selzer ME. 2013; Neuroplasticity in the spinal cord. Handb Clin Neurol. 110:23–42. DOI: 10.1016/B978-0-444-52901-5.00003-4. PMID: 23312628.50. Anjum A, Yazid MD, Daud MF, Idris J, Hwei Ng AM, Naicker AS, et al. 2020; Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 21:7533. DOI: 10.3390/ijms21207533. PMID: 33066029. PMCID: PMC7589539. PMID: f43bbee496ef470c8c47ae69560cb4fd.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spinal Cord Regeneration in Rat using Neural Stem Cell Differentiated from Human Telencephalon
- Intrathecal administration of naringenin improves motor dysfunction and neuropathic pain following compression spinal cord injury in rats: relevance to its antioxidant and anti-inflammatory activities
- The Role of Human Neural Stem Cell Secretomes on the Repair of Spinal Cord Injury Post-laminectomy in Rattus norvegicus Through the Analysis of Basso–Beattie–Bresnahan Score Locomotors, Interleukin-10, Matrix Metalloproteinase 9, and Transforming Growth Factor-β
- Synergistic interaction between acetaminophen and L-carnosine improved neuropathic pain via NF-κB pathway and antioxidant properties in chronic constriction injury model
- Transplantation of PSA-NCAM-Positive Neural Precursors from Human Embryonic Stem Cells Promotes Functional Recovery in an Animal Model of Spinal Cord Injury