Korean J Physiol Pharmacol.
2023 Jan;27(1):85-94. 10.4196/kjpp.2023.27.1.85.
Age-dependent expression of ion channel genes in rat
- Affiliations
-
- 1Department of Physiology, School of Medicine, Jeju National University, Jeju 63243, Korea
- 2Department of Physiology and Cell Biology, University of Nevada, Reno School of Medicine, Reno, NV 89557, USA
- KMID: 2537506
- DOI: http://doi.org/10.4196/kjpp.2023.27.1.85
Abstract
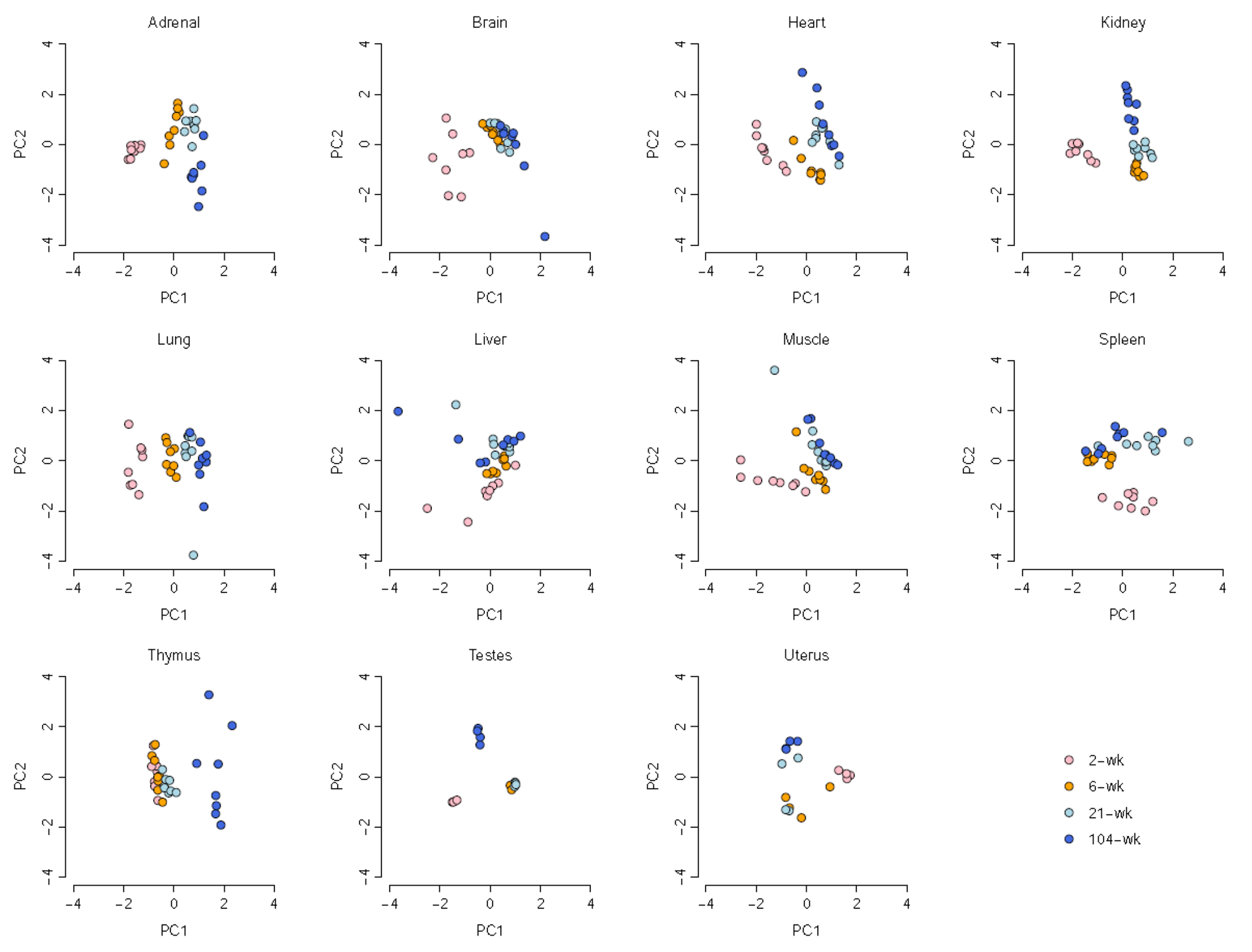
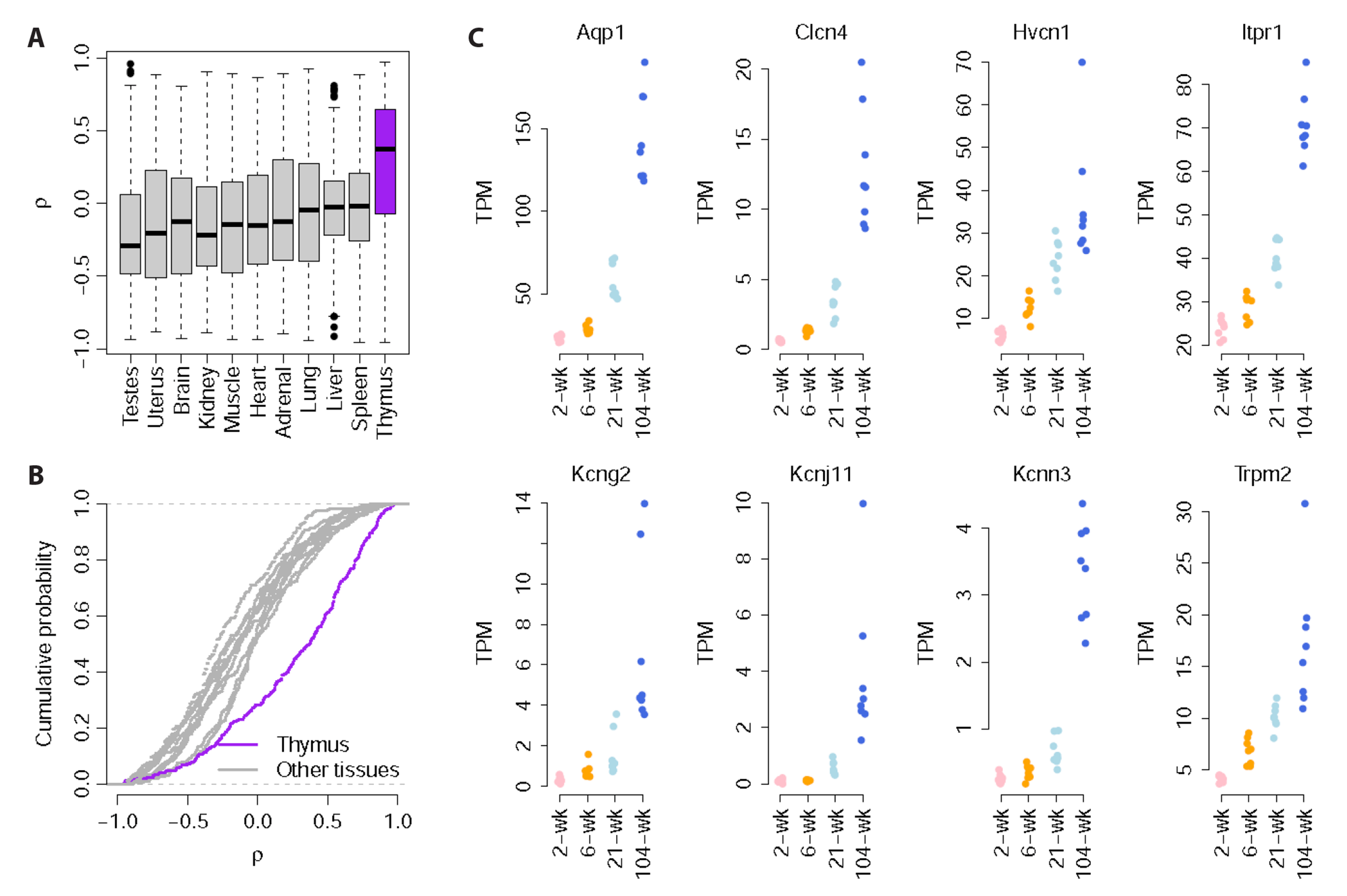
- Ion channels regulate a large number of cellular functions and their functional role in many diseases makes them potential therapeutic targets. Given their diverse distribution across multiple organs, the roles of ion channels, particularly in age-associated transcriptomic changes in specific organs, are yet to be fully revealed. Using RNA-seq data, we investigated the rat transcriptomic profiles of ion channel genes across 11 organs/tissues and 4 developmental stages in both sexes of Fischer 344 rats and identify tissue-specific and age-dependent changes in ion channel gene expression. Organ-enriched ion channel genes were identified. In particular, the brain showed higher tissue-specificity of ion channel genes, including Gabrd, Gabra6, Gabrg2, Grin2a, and Grin2b. Notably, age-dependent changes in ion channel gene expression were prominently observed in the thymus, including in Aqp1, Clcn4, Hvcn1, Itpr1, Kcng2, Kcnj11, Kcnn3, and Trpm2. Our comprehensive study of ion channel gene expression will serve as a primary resource for biological studies of aging-related diseases caused by abnormal ion channel functions.
Keyword
Figure
Reference
-
1. Han K, Jin X, Guo X, Cao G, Tian S, Song Y, Zuo Y, Yu P, Gao G, Chang YZ. 2021; Nrf2 knockout altered brain iron deposition and mitigated age-related motor dysfunction in aging mice. Free Radic Biol Med. 162:592–602. DOI: 10.1016/j.freeradbiomed.2020.11.019. PMID: 33248265.2. Tellez JO, Mczewski M, Yanni J, Sutyagin P, Mackiewicz U, Atkinson A, Inada S, Beresewicz A, Billeter R, Dobrzynski H, Boyett MR. 2011; Ageing-dependent remodelling of ion channel and Ca2+ clock genes underlying sino-atrial node pacemaking. Exp Physiol. 96:1163–1178. DOI: 10.1113/expphysiol.2011.057752. PMID: 21724736.3. Melé M, Ferreira PG, Reverter F, DeLuca DS, Monlong J, Sammeth M, Young TR, Goldmann JM, Pervouchine DD, Sullivan TJ, Johnson R, Segrè AV, Djebali S, Niarchou A, Wright FA, Lappalainen T, Calvo M, Getz G, Dermitzakis ET, et al. GTEx Consortium. 2015; Human genomics. The human transcriptome across tissues and individuals. Science. 348:660–665. DOI: 10.1126/science.aaa0355. PMID: 25954002. PMCID: PMC4547472. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84929015296&origin=inward.4. Bodyak N, Kang PM, Hiromura M, Sulijoadikusumo I, Horikoshi N, Khrapko K, Usheva A. 2002; Gene expression profiling of the aging mouse cardiac myocytes. Nucleic Acids Res. 30:3788–3794. DOI: 10.1093/nar/gkf497. PMID: 12202764. PMCID: PMC137419. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036713499&origin=inward.5. Zhao L, Li Z, Vong JSL, Chen X, Lai HM, Yan LYC, Huang J, Sy SKH, Tian X, Huang Y, Chan HYE, So HC, Ng WL, Tang Y, Lin WJ, Mok VCT, Ko H. 2020; Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat Commun. 11:4413. DOI: 10.1038/s41467-020-18249-3. PMID: 32887883. PMCID: PMC7474063. PMID: 0cd6b88e4d0b4038b2b9807cac7e8608. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85090286333&origin=inward.6. Showmaker KC, Cobb MB, Johnson AC, Yang W, Garrett MR. 2020; Whole genome sequencing and novel candidate genes for CAKUT and altered nephrogenesis in the HSRA rat. Physiol Genomics. 52:56–70. DOI: 10.1152/physiolgenomics.00112.2019. PMID: 31841396. PMCID: PMC6985787. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85078551518&origin=inward.7. Yu Y, Fuscoe JC, Zhao C, Guo C, Jia M, Qing T, Bannon DI, Lancashire L, Bao W, Du T, Luo H, Su Z, Jones WD, Moland CL, Branham WS, Qian F, Ning B, Li Y, Hong H, Guo L, et al. 2014; A rat RNA-Seq transcriptomic BodyMap across 11 organs and 4 developmental stages. Nat Commun. 5:3230. DOI: 10.1038/ncomms4230. PMID: 24510058. PMCID: PMC3926002. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84907492839&origin=inward.8. Zhou T, Xie X, Li M, Shi J, Zhou JJ, Knox KS, Wang T, Chen Q, Gu W. 2018; Rat BodyMap transcriptomes reveal unique circular RNA features across tissue types and developmental stages. RNA. 24:1443–1456. DOI: 10.1261/rna.067132.118. PMID: 30093490. PMCID: PMC6191709. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85055073493&origin=inward.9. Yu Y, Zhao C, Su Z, Wang C, Fuscoe JC, Tong W, Shi L. 2014; Comprehensive RNA-Seq transcriptomic profiling across 11 organs, 4 ages, and 2 sexes of Fischer 344 rats. Sci Data. 1:140013. DOI: 10.1038/sdata.2014.13. PMID: 25977771. PMCID: PMC4381750. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84960885434&origin=inward.10. Li M, Xie X, Zhou J, Sheng M, Yin X, Ko EA, Zhou T, Gu W. 2017; Quantifying circular RNA expression from RNA-Seq data using model-based framework. Bioinformatics. 33:2131–2139. DOI: 10.1093/bioinformatics/btx129. PMID: 28334396. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85020877053&origin=inward.11. Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A. 2013; NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 41:D991–D995. DOI: 10.1093/nar/gks1193. PMID: 23193258. PMCID: PMC3531084. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84874271270&origin=inward.12. DeLuca DS, Levin JZ, Sivachenko A, Fennell T, Nazaire MD, Williams C, Reich M, Winckler W, Getz G. 2012; RNA-SeQC: RNA-Seq metrics for quality control and process optimization. Bioinformatics. 28:1530–1532. DOI: 10.1093/bioinformatics/bts196. PMID: 22539670. PMCID: PMC3356847. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84861743958&origin=inward.13. Cunningham F, Amode MR, Barrell D, Beal K, Billis K, Brent S, Carvalho-Silva D, Clapham P, Coates G, Fitzgerald S, Gil L, Girón CG, Gordon L, Hourlier T, Hunt SE, Janacek SH, Johnson N, Juettemann T, Kähäri AK, Keenan S, et al. 2015; Ensembl 2015. Nucleic Acids Res. 43:D662–D669. DOI: 10.1093/nar/gku1010. PMID: 25352552. PMCID: PMC4383879. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84946037477&origin=inward.14. Kim YW, Ko EA, Jung SC, Lee D, Seo Y, Kim S, Kim JH, Bang H, Zhou T, Ko JH. 2021; Transcriptomic insight into the translational value of two murine models in human atopic dermatitis. Sci Rep. 23:6616. DOI: 10.1038/s41598-021-86049-w. PMID: 33758305. PMCID: PMC7988112. PMID: 1c49ef5e932a4699b0cb9617720251bb. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85103202278&origin=inward.15. Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, Bar-Even A, Horn-Saban S, Safran M, Domany E, Lancet D, Shmueli O. 2005; Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics. 21:650–659. DOI: 10.1093/bioinformatics/bti042. PMID: 15388519. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=20144376345&origin=inward.16. Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Southan C, Sharman JL, Campo B, Cavanagh DR, Alexander SPH, Davenport AP, Spedding M, Davies JA. 2020; The IUPHAR/BPS guide to PHARMACOLOGY in 2020: extending IMMUNOPHARMACOLOGY content and introducing the IUPHAR/MMV guide to Malaria PHARMACOLOGY. Nucleic Acids Res. 48:D1006–D1021. DOI: 10.1093/nar/gkz951. PMID: 31691834. PMCID: PMC7145572. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85077012757&origin=inward.17. Petroff OA. 2002; GABA and glutamate in the human brain. Neuroscientist. 8:562–573. DOI: 10.1177/1073858402238515. PMID: 12467378. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036892023&origin=inward.18. Su W, Cao R, Zhang XY, Guan Y. 2020; Aquaporins in the kidney: physiology and pathophysiology. Am J Physiol Renal Physiol. 318:F193–F203. DOI: 10.1152/ajprenal.00304.2019. PMID: 31682170. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85077761313&origin=inward.19. Sun XH, Zhu YY, Wang L, Liu HL, Ling Y, Li ZL, Sun LB. 2017; The Catsper channel and its roles in male fertility: a systematic review. Reprod Biol Endocrinol. 15:65. DOI: 10.1186/s12958-017-0281-2. PMID: 28810916. PMCID: PMC5558725. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85028062232&origin=inward.20. Wang R, Gurguis CI, Gu W, Ko EA, Lim I, Bang H, Zhou T, Ko JH. 2015; Ion channel gene expression predicts survival in glioma patients. Sci Rep. 5:11593. DOI: 10.1038/srep11593. PMID: 26235283. PMCID: PMC4522676. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84938517742&origin=inward.21. Hamilton S, Terentyev D. 2019; Altered intracellular calcium homeostasis and arrhythmogenesis in the aged heart. Int J Mol Sci. 20:2386. DOI: 10.3390/ijms20102386. PMID: 31091723. PMCID: PMC6566636. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85066853743&origin=inward.22. Ahn C, Choi JS, Jeung EB. 2018; Organ-specific expression of the divalent ion channel proteins NCKX3, TRPV2, CTR1, ATP7A, IREG1 and HEPH in various canine organs. Mol Med Rep. 18:1773–1781. DOI: 10.3892/mmr.2018.9148. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85049576225&origin=inward.23. Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. 2005; Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet. 14:3035–3046. DOI: 10.1093/hmg/ddi336. PMID: 16155113. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=27544503765&origin=inward.24. Hirata Y, Nomura K, Kato D, Tachibana Y, Niikura T, Uchiyama K, Hosooka T, Fukui T, Oe K, Kuroda R, Hara Y, Adachi T, Shibasaki K, Wake H, Ogawa W. 2022; A Piezo1/KLF15/IL-6 axis mediates immobilization-induced muscle atrophy. J Clin Invest. 132:1–13. DOI: 10.1172/JCI154611. PMID: 35290243. PMCID: PMC9159676. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85130190069&origin=inward.25. Kiselyov KK, Ahuja M, Rybalchenko V, Patel S, Muallem S. 2012; The intracellular Ca²+ channels of membrane traffic. Channels (Austin). 6:344–351. DOI: 10.4161/chan.21723. PMID: 22907062. PMCID: PMC3508773. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84868140120&origin=inward.26. Kaufmann U, Shaw PJ, Kozhaya L, Subramanian R, Gaida K, Unutmaz D, McBride HJ, Feske S. 2016; Selective ORAI1 inhibition ameliorates autoimmune central nervous system inflammation by suppressing effector but not regulatory T cell function. J Immunol. 196:573–585. DOI: 10.4049/jimmunol.1501406. PMID: 26673135. PMCID: PMC4707123. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84954118152&origin=inward.27. Zöphel D, Hof C, Lis A. 2020; Altered Ca2+ homeostasis in immune cells during aging: role of ion channels. Int J Mol Sci. 22:110. DOI: 10.3390/ijms22010110. PMID: 33374304. PMCID: PMC7794837. PMID: 3064d0972f914f42bfef8ef8fdd9f824. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85098703184&origin=inward.28. Guzik TJ, Touyz RM. 2017; Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 70:660–667. DOI: 10.1161/HYPERTENSIONAHA.117.07802. PMID: 28784646. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030998179&origin=inward.29. Harries LW, Hernandez D, Henley W, Wood AR, Holly AC, Bradley-Smith RM, Yaghootkar H, Dutta A, Murray A, Frayling TM, Guralnik JM, Bandinelli S, Singleton A, Ferrucci L, Melzer D. 2011; Human aging is characterized by focused changes in gene expression and deregulation of alternative splicing. Aging Cell. 10:868–878. DOI: 10.1111/j.1474-9726.2011.00726.x. PMID: 21668623. PMCID: PMC3173580. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=80052803450&origin=inward.30. Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R, Davis RW, Becker KG, Owen AB, Kim SK. 2006; Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2:e115. DOI: 10.1371/journal.pgen.0020115. PMCID: PMC1513263. PMID: 16789832. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33746621756&origin=inward.31. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013; The hallmarks of aging. Cell. 153:1194–1217. DOI: 10.1016/j.cell.2013.05.039. PMID: 23746838. PMCID: PMC3836174. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84878864199&origin=inward.32. Viñuela A, Brown AA, Buil A, Tsai PC, Davies MN, Bell JT, Dermitzakis ET, Spector TD, Small KS. 2018; Age-dependent changes in mean and variance of gene expression across tissues in a twin cohort. Hum Mol Genet. 27:732–741. DOI: 10.1093/hmg/ddx424. PMID: 29228364. PMCID: PMC5886097. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85041516302&origin=inward.33. DiFranco M, Yu C, Quiñonez M, Vergara JL. 2013; Age-dependent chloride channel expression in skeletal muscle fibres of normal and HSALR myotonic mice. J Physiol. 591:1347–1371. DOI: 10.1113/jphysiol.2012.246546. PMID: 23247112. PMCID: PMC3607876. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84874428820&origin=inward.34. Kakae M, Miyanohara J, Morishima M, Nagayasu K, Mori Y, Shirakawa H, Kaneko S. 2019; Pathophysiological role of TRPM2 in age-related cognitive impairment in mice. Neuroscience. 408:204–213. DOI: 10.1016/j.neuroscience.2019.04.012. PMID: 30999030. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064867322&origin=inward.35. Camandola S, Mattson MP. 2011; Aberrant subcellular neuronal calcium regulation in aging and Alzheimer's disease. Biochim Biophys Acta. 1813:965–973. DOI: 10.1016/j.bbamcr.2010.10.005. PMID: 20950656. PMCID: PMC3032815. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79955665348&origin=inward.36. Trombetta-Lima M, Krabbendam IE, Dolga AM. 2020; Calcium-activated potassium channels: implications for aging and age-related neurodegeneration. Int J Biochem Cell Biol. 123:105748. DOI: 10.1016/j.biocel.2020.105748. PMID: 32353429. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85084943207&origin=inward.37. Geigerseder C, Doepner R, Thalhammer A, Frungieri MB, Gamel-Didelon K, Calandra RS, Köhn FM, Mayerhofer A. 2003; Evidence for a GABAergic system in rodent and human testis: local GABA production and GABA receptors. Neuroendocrinology. 77:314–323. DOI: 10.1159/000070897. PMID: 12806177. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0038010291&origin=inward.38. Tian J, Lu Y, Zhang H, Chau CH, Dang HN, Kaufman DL. 2004; Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 173:5298–5304. DOI: 10.4049/jimmunol.173.8.5298. PMID: 15470076. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=6344285328&origin=inward.39. Balan S, Sathyan S, Radha SK, Joseph V, Radhakrishnan K, Banerjee M. 2013; GABRG2, rs211037 is associated with epilepsy susceptibility, but not with antiepileptic drug resistance and febrile seizures. Pharmacogenet Genomics. 23:605–610. DOI: 10.1097/FPC.0000000000000000. PMID: 24061200. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84886601313&origin=inward.40. Kumar P, Sharma D. 2020; Ameliorative effect of curcumin on altered expression of CACNA1A and GABRD in the pathogenesis of FeCl3-induced epilepsy. Mol Biol Rep. 47:5699–5710. DOI: 10.1007/s11033-020-05538-9. PMID: 32803504. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089458405&origin=inward.41. Haerian BS, Baum L, Kwan P, Cherny SS, Shin JG, Kim SE, Han BG, Tan HJ, Raymond AA, Tan CT, Mohamed Z. 2016; Contribution of GABRG2 polymorphisms to risk of epilepsy and febrile seizure: a multicenter cohort study and meta-analysis. Mol Neurobiol. 53:5457–5467. DOI: 10.1007/s12035-015-9457-y. PMID: 26452361. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84944705310&origin=inward.42. Zhang H, Zhang L, Tang Y, Wang C, Chen Y, Shu J, Zhang K. 2019; Systemic screening identifies GABRD, a subunit gene of GABAA receptor as a prognostic marker in adult IDH wild-type diffuse low-grade glioma. Biomed Pharmacother. 118:109215. DOI: 10.1016/j.biopha.2019.109215. PMID: 31545245. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85070984585&origin=inward.43. Sarathi A, Palaniappan A. 2019; Novel significant stage-specific differentially expressed genes in hepatocellular carcinoma. BMC Cancer. 19:663. DOI: 10.1186/s12885-019-5838-3. PMID: 31277598. PMCID: PMC6612102. PMID: 6f78d393eebc4b259e146be696ab6389. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85069267765&origin=inward.44. Wu M, Kim KY, Park WC, Ryu HS, Choi SC, Kim MS, et al. 2020; Enhanced expression of GABRD predicts poor prognosis in patients with colon adenocarcinoma. Transl Oncol. 13:100861. DOI: 10.1016/j.tranon.2020.100861. PMID: 32891902. PMCID: PMC7484591. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85090244339&origin=inward.45. Myers SJ, Yuan H, Kang JQ, Tan FCK, Traynelis SF, Low CM. 2019; Distinct roles of GRIN2A and GRIN2B variants in neurological conditions. F1000Res. 8:F1000 Faculty Rev-1940. DOI: 10.12688/f1000research.18949.1. PMID: 31807283. PMCID: PMC6871362. PMID: 01db0138bf5048dbb3442018b9248bcd. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85076171438&origin=inward.46. Endele S, Rosenberger G, Geider K, Popp B, Tamer C, Stefanova I, Milh M, Kortüm F, Fritsch A, Pientka FK, Hellenbroich Y, Kalscheuer VM, Kohlhase J, Moog U, Rappold G, Rauch A, Ropers HH, von Spiczak S, Tönnies H, Villeneuve N, et al. 2010; Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat Genet. 42:1021–1026. DOI: 10.1038/ng.677. PMID: 20890276. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78049329316&origin=inward.47. Comelli M, Meo M, Cervantes DO, Pizzo E, Plosker A, Mohler PJ, Hund TJ, Jacobson JT, Meste O, Rota M. 2020; Rhythm dynamics of the aging heart: an experimental study using conscious, restrained mice. Am J Physiol Heart Circ Physiol. 319:H893–H905. DOI: 10.1152/ajpheart.00379.2020. PMID: 32886003. PMCID: PMC7654658. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85092749808&origin=inward.48. Benkert J, Hess S, Roy S, Beccano-Kelly D, Wiederspohn N, Duda J, Simons C, Patil K, Gaifullina A, Mannal N, Dragicevic E, Spaich D, Müller S, Nemeth J, Hollmann H, Deuter N, Mousba Y, Kubisch C, Poetschke C, Striessnig J, et al. 2019; Cav2.3 channels contribute to dopaminergic neuron loss in a model of Parkinson's disease. Nat Commun. 10:5094. DOI: 10.1038/s41467-019-12834-x. PMID: 31704946. PMCID: PMC6841684. PMID: 8271fb3221094175818e53f6966e1e51. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85074714711&origin=inward.49. Signore S, Sorrentino A, Borghetti G, Cannata A, Meo M, Zhou Y, Kannappan R, Pasqualini F, O'Malley H, Sundman M, Tsigkas N, Zhang E, Arranto C, Mangiaracina C, Isobe K, Sena BF, Kim J, Goichberg P, Nahrendorf M, Isom LL, et al. 2015; Late Na+ current and protracted electrical recovery are critical determinants of the aging myopathy. Nat Commun. 6:8803. DOI: 10.1038/ncomms9803. PMID: 26541940. PMCID: PMC4638135. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84946599747&origin=inward.50. Rice RA, Berchtold NC, Cotman CW, Green KN. 2014; Age-related downregulation of the CaV3.1 T-type calcium channel as a mediator of amyloid beta production. Neurobiol Aging. 35:1002–1011. DOI: 10.1016/j.neurobiolaging.2013.10.090. PMID: 24268883. PMCID: PMC3939046. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84893814237&origin=inward.51. Ye H, Jalini S, Mylvaganam S, Carlen P. 2010; Activation of large-conductance Ca2+-activated K+ channels depresses basal synaptic transmission in the hippocampal CA1 area in APP (swe/ind) TgCRND8 mice. Neurobiol Aging. 31:591–604. DOI: 10.1016/j.neurobiolaging.2008.05.012. PMID: 18547679. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77049103601&origin=inward.52. Lustig A, Carter A, Bertak D, Enika D, Vandanmagsar B, Wood W, Becker KG, Weeraratna AT, Taub DD. 2009; Transcriptome analysis of murine thymocytes reveals age-associated changes in thymic gene expression. Int J Med Sci. 6:51–64. DOI: 10.7150/ijms.6.51. PMID: 19214242. PMCID: PMC2640475. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=60049090034&origin=inward.53. Aw D, Taylor-Brown F, Cooper K, Palmer DB. 2009; Phenotypical and morphological changes in the thymic microenvironment from ageing mice. Biogerontology. 10:311–322. DOI: 10.1007/s10522-008-9182-2. PMID: 18931936. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=67349171030&origin=inward.54. Gui J, Zhu X, Dohkan J, Cheng L, Barnes PF, Su DM. 2007; The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. Int Immunol. 19:1201–1211. DOI: 10.1093/intimm/dxm095. PMID: 17804689. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34848922760&origin=inward.55. Ki S, Park D, Selden HJ, Seita J, Chung H, Kim J, Iyer VR, Ehrlich LIR. 2014; Global transcriptional profiling reveals distinct functions of thymic stromal subsets and age-related changes during thymic involution. Cell Rep. 9:402–415. DOI: 10.1016/j.celrep.2014.08.070. PMID: 25284794. PMCID: PMC4194175. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84907963337&origin=inward.56. Blank T, Nijholt I, Kye MJ, Radulovic J, Spiess J. 2003; Small-conductance, Ca2+-activated K+ channel SK3 generates age-related memory and LTP deficits. Nat Neurosci. 6:911–912. DOI: 10.1038/nn1101. PMID: 12883553. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0042861455&origin=inward.57. Jacobsen JP, Redrobe JP, Hansen HH, Petersen S, Bond CT, Adelman JP, Mikkelsen JD, Mirza NR. 2009; Selective cognitive deficits and reduced hippocampal brain-derived neurotrophic factor mRNA expression in small-conductance calcium-activated K+ channel deficient mice. Neuroscience. 163:73–81. DOI: 10.1016/j.neuroscience.2009.05.062. PMID: 19482064. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=68349144706&origin=inward.58. Khan V, Verma AK, Bhatt D, Khan S, Hasan R, Goyal Y, Ramachandran S, Alsahli MA, Rahmani AH, Almatroudi A, Shareef MY, Meena B, Dev K. 2020; Association of genetic variants of KCNJ11 and KCNQ1 genes with risk of type 2 diabetes mellitus (T2DM) in the Indian population: a case-control study. Int J Endocrinol. 2020:5924756. DOI: 10.1155/2020/5924756. PMID: 33101408. PMCID: PMC7569458. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85094644340&origin=inward.59. Jablonski EM, Webb AN, McConnell NA, Riley MC, Hughes FM Jr. 2004; Plasma membrane aquaporin activity can affect the rate of apoptosis but is inhibited after apoptotic volume decrease. Am J Physiol Cell Physiol. 286:C975–C985. DOI: 10.1152/ajpcell.00180.2003. PMID: 14644770. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=2142703737&origin=inward.60. Choi JY, Hwang CY, Lee B, Lee SM, Bahn YJ, Lee KP, Kang M, Kim YS, Woo SH, Lim JY, Kim E, Kwon KS. 2016; Age-associated repression of type 1 inositol 1, 4, 5-triphosphate receptor impairs muscle regeneration. Aging (Albany NY). 8:2062–2080. DOI: 10.18632/aging.101039. PMID: 27658230. PMCID: PMC5076452. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84991494501&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Voltage-dependent Na+ Channels in the Albino Guinea Pig Cochlea
- Expression of potassium channel genes predicts clinical outcome in lung cancer
- The expression of voltage-dependent K+ channels in stria vascularis of the guinea pig cochlea
- Expression of ATP-sensitive potassium channel and sulfonylurea receptor in neonate and adult rat tissues
- Genetics of Channelopathy: Familial Periodic Paralysis