Korean J Physiol Pharmacol.
2023 Jan;27(1):49-59. 10.4196/kjpp.2023.27.1.49.
Association between metabolic syndrome components and cardiac autonomic modulation in southern Indian adults with pre-metabolic syndrome: hyperglycemia is the major contributing factor
- Affiliations
-
- 1Department of Physiology, Sri Siddhartha Institute of Medical Sciences & Research Centre, Bangalore 562123, India
- 2Department of Physiology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry 605008, India
- 3Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry 605008, India
- 4Department of Biochemistry, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry 605008, India
- 5Department of Dermatology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry 605008, India
- KMID: 2537503
- DOI: http://doi.org/10.4196/kjpp.2023.27.1.49
Abstract
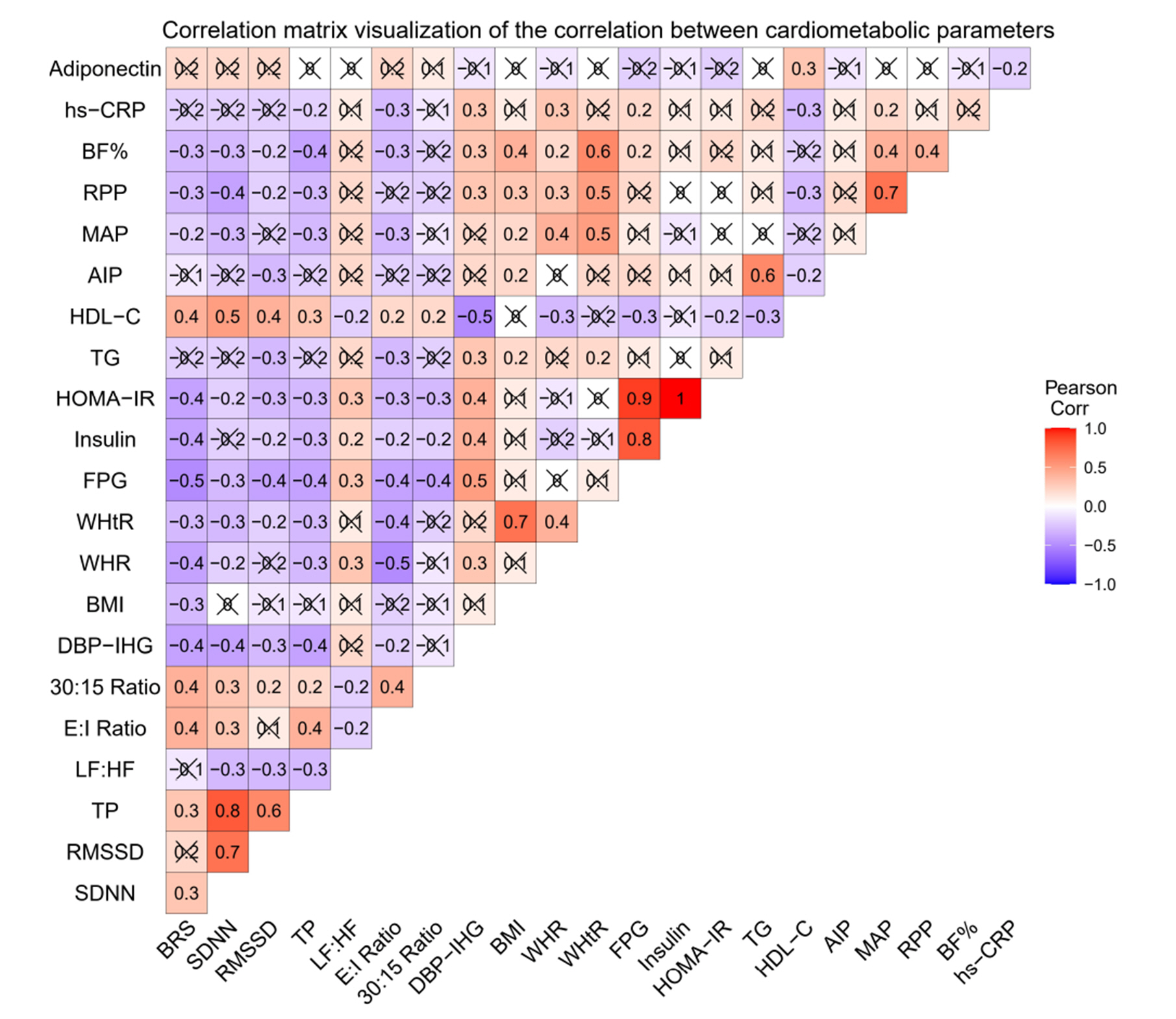
- Metabolic syndrome (MetS) involves multi-factorial conditions linked to an elevated risk of type 2 diabetes mellitus and cardiovascular disease. Pre-metabolic syndrome (pre-MetS) possesses two MetS components but does not meet the MetS diagnostic criteria. Although cardiac autonomic derangements are evident in MetS, there is little information on their status in pre-MetS subjects. In this study, we sought to examine cardiac autonomic functions in pre-MetS and to determine which MetS component is more responsible for impaired cardiac autonomic functions. A total of 182 subjects were recruited and divided into healthy controls (n=89) and pre-MetS subjects (n=93) based on inclusion and exclusion criteria. We performed biochemical profiles on fasting blood samples to detect pre-MetS. Using standardized protocols, we evaluated anthropometric data, body composition, baroreflex sensitivity (BRS), heart rate variability (HRV), and autonomic function tests (AFTs). We further examined these parameters in pre-MetS subjects for each MetS component. Compared to healthy controls, we observed a significant cardiac autonomic dysfunction (CAD) through reduced BRS, lower overall HRV, and altered AFT parameters in pre-MetS subjects, accompanied by markedly varied anthropometric, clinical and biochemical parameters. Furthermore, all examined BRS, HRV, and AFT parameters exhibited an abnormal trend and significant correlation toward hyperglycemia. This study demonstrates CAD in pre-MetS subjects with reduced BRS, lower overall HRV, and altered AFT parameters. Hyperglycemia was considered an independent determinant of alterations in all the examined BRS, HRV, and AFT parameters. Thus, hyperglycemia may contribute to CAD in pre-MetS subjects before progressing to MetS.
Keyword
Figure
Reference
-
1. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. 2004; Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 109:433–438. DOI: 10.1161/01.CIR.0000111245.75752.C6. PMID: 14744958. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0842277242&origin=inward.2. Samson SL, Garber AJ. 2014; Metabolic syndrome. Endocrinol Metab Clin North Am. 43:1–23. DOI: 10.1016/j.ecl.2013.09.009. PMID: 24582089. PMCID: PMC5095229. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84896840279&origin=inward.3. McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, Ballantyne CM, Heiss G. 2005; The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 28:385–390. DOI: 10.2337/diacare.28.2.385. PMID: 15677797. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=12844262139&origin=inward.4. Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. 2008; The metabolic syndrome. Endocr Rev. 29:777–822. DOI: 10.1210/er.2008-0024. PMID: 18971485. PMCID: PMC5393149. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=57349096045&origin=inward.5. Alberti KG, Zimmet P, Shaw J. 2005; The metabolic syndrome--a new worldwide definition. Lancet. 366:1059–1062. DOI: 10.1016/S0140-6736(05)67402-8. PMID: 16182882. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=25144459980&origin=inward.6. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. 2015; Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 313:1973–1974. DOI: 10.1001/jama.2015.4260. PMID: 25988468. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84929501636&origin=inward.7. Stagnaro S. 2007; Epidemiological evidence for the non-random clustering of the components of the metabolic syndrome: multicentre study of the Mediterranean Group for the Study of Diabetes. Eur J Clin Nutr. 61:1143–1144. DOI: 10.1038/sj.ejcn.1602668. PMID: 17299468. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34548583506&origin=inward.8. Yin Q, Chen X, Li L, Zhou R, Huang J, Yang D. 2013; Apolipoprotein B/apolipoprotein A1 ratio is a good predictive marker of metabolic syndrome and pre-metabolic syndrome in Chinese adolescent women with polycystic ovary syndrome. J Obstet Gynaecol Res. 39:203–209. DOI: 10.1111/j.1447-0756.2012.01907.x. PMID: 22672648. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84875738636&origin=inward.9. Thayer JF, Yamamoto SS, Brosschot JF. 2010; The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 141:122–131. DOI: 10.1016/j.ijcard.2009.09.543. PMID: 19910061. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77952241896&origin=inward.10. Stuckey MI, Tulppo MP, Kiviniemi AM, Petrella RJ. 2014; Heart rate variability and the metabolic syndrome: a systematic review of the literature. Diabetes Metab Res Rev. 30:784–793. DOI: 10.1002/dmrr.2555. PMID: 24816921. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84910662918&origin=inward.11. Wulsin LR, Horn PS, Perry JL, Massaro JM, D'Agostino RB Sr. 2016; The contribution of autonomic imbalance to the development of metabolic syndrome. Psychosom Med. 78:474–480. DOI: 10.1097/PSY.0000000000000290. PMID: 26716816. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84952685378&origin=inward.12. Endukuru CK, Gaur GS, Yerrabelli D, Sahoo J, Vairappan B. 2020; Impaired baroreflex sensitivity and cardiac autonomic functions are associated with cardiovascular disease risk factors among patients with metabolic syndrome in a tertiary care teaching hospital of South-India. Diabetes Metab Syndr. 14:2043–2051. DOI: 10.1016/j.dsx.2020.10.018. PMID: 33113471. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85093648588&origin=inward.13. Jarczok MN, Li J, Mauss D, Fischer JE, Thayer JF. 2013; Heart rate variability is associated with glycemic status after controlling for components of the metabolic syndrome. Int J Cardiol. 167:855–861. DOI: 10.1016/j.ijcard.2012.02.002. PMID: 22386703. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84880917025&origin=inward.14. Shahani BT, Day TJ, Cros D, Khalil N, Kneebone CS. 1990; RR interval variation and the sympathetic skin response in the assessment of autonomic function in peripheral neuropathy. Arch Neurol. 47:659–664. DOI: 10.1001/archneur.1990.00530060069021. PMID: 2161208. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0025339359&origin=inward.15. Kudaiberdieva G, Görenek B, Timuralp B. 2007; Heart rate variability as a predictor of sudden cardiac death. Anadolu Kardiyol Derg. 7 Suppl 1:68–70. PMID: 17584685. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34447109483&origin=inward.16. Wulsin LR, Horn PS, Perry JL, Massaro JM, D'Agostino RB. 2015; Autonomic imbalance as a predictor of metabolic risks, cardiovascular disease, diabetes, and mortality. J Clin Endocrinol Metab. 100:2443–2448. DOI: 10.1210/jc.2015-1748. PMID: 26047073. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84930813947&origin=inward.17. Imholz BP, Wieling W, van Montfrans GA, Wesseling KH. 1998; Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res. 38:605–616. DOI: 10.1016/S0008-6363(98)00067-4. PMID: 9747429. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032102644&origin=inward.18. Skrapari I, Tentolouris N, Katsilambros N. 2006; Baroreflex function: determinants in healthy subjects and disturbances in diabetes, obesity and metabolic syndrome. Curr Diabetes Rev. 2:329–338. DOI: 10.2174/157339906777950589. PMID: 18220637. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33746912406&origin=inward.19. Callaghan BC, Xia R, Banerjee M, de Rekeneire N, Harris TB, Newman AB, Satterfield S, Schwartz AV, Vinik AI, Feldman EL, Strotmeyer ES. 2016; Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care. 39:801–807. DOI: 10.2337/dc16-0081. PMID: 26965720. PMCID: PMC4839175. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84964757867&origin=inward.20. Assoumou HG, Pichot V, Barthelemy JC, Dauphinot V, Celle S, Gosse P, Kossovsky M, Gaspoz JM, Roche F. 2010; Metabolic syndrome and short-term and long-term heart rate variability in elderly free of clinical cardiovascular disease: the PROOF study. Rejuvenation Res. 13:653–663. DOI: 10.1089/rej.2010.1019. PMID: 20818933. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78651273905&origin=inward.21. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. 2009; Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 120:1640–1645. DOI: 10.1161/CIRCULATIONAHA.109.192644. PMID: 19805654. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70350245011&origin=inward.22. World Health Organization. 1995. Physical status: the use of and interpretation of anthropometry, report of a WHO expert committee. World Health Organization;Geneva: https://apps.who.int/iris/handle/10665/37003. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34447109483&origin=inward.23. Garrow JS, Webster J. 1985; Quetelet's index (W/H2) as a measure of fatness. Int J Obes. 9:147–153. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34447109483&origin=inward.24. Fukuyama N, Homma K, Wakana N, Kudo K, Suyama A, Ohazama H, Tsuji C, Ishiwata K, Eguchi Y, Nakazawa H, Tanaka E. 2008; Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 43:1–5. DOI: 10.3164/jcbn.2008036. PMID: 18648653. PMCID: PMC2459246. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=50349085281&origin=inward.25. Heart rate variability. 1996; Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 17:354–381. PMID: 8737210. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029988782&origin=inward.26. White WB. 1999; Heart rate and the rate-pressure product as determinants of cardiovascular risk in patients with hypertension. Am J Hypertens. 12(2 Pt 2):50S–55S. DOI: 10.1016/S0895-7061(98)00280-5. PMID: 10090295.27. Novak P. 2011; Quantitative autonomic testing. J Vis Exp. (53):2502. DOI: 10.3791/2502. PMID: 21788940. PMCID: PMC3196175. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=80355131581&origin=inward.28. Ewing DJ, Martyn CN, Young RJ, Clarke BF. 1985; The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 8:491–498. DOI: 10.2337/diacare.8.5.491. PMID: 4053936. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0021984563&origin=inward.29. Kurl S, Laaksonen DE, Jae SY, Mäkikallio TH, Zaccardi F, Kauhanen J, Ronkainen K, Laukkanen JA. 2016; Metabolic syndrome and the risk of sudden cardiac death in middle-aged men. Int J Cardiol. 203:792–797. DOI: 10.1016/j.ijcard.2015.10.218. PMID: 26595786. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84952684375&origin=inward.30. Grassi G, Seravalle G. 2006; Autonomic imbalance and metabolic syndrome: unravelling interactions, mechanisms and outcomes. J Hypertens. 24:47–49. DOI: 10.1097/01.hjh.0000198040.47128.4c. PMID: 16331100. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=29144486187&origin=inward.31. Ashwell M, Gunn P, Gibson S. 2012; Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 13:275–286. DOI: 10.1111/j.1467-789X.2011.00952.x. PMID: 22106927. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84857194676&origin=inward.32. Lee JF, Harrison ML, Christmas KM, Kim K, Hurr C, Brothers RM. 2014; Elevated resting heart rate and reduced orthostatic tolerance in obese humans. Clin Auton Res. 24:39–46. DOI: 10.1007/s10286-013-0222-x. PMID: 24292891. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84894276783&origin=inward.33. Pikkujämsä SM, Huikuri HV, Airaksinen KE, Rantala AO, Kauma H, Lilja M, Savolainen MJ, Kesäniemi YA. 1998; Heart rate variability and baroreflex sensitivity in hypertensive subjects with and without metabolic features of insulin resistance syndrome. Am J Hypertens. 11:523–531. DOI: 10.1016/S0895-7061(98)00035-1. PMID: 9633787. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0345164360&origin=inward.34. Lindgren K, Hagelin E, Hansén N, Lind L. 2006; Baroreceptor sensitivity is impaired in elderly subjects with metabolic syndrome and insulin resistance. J Hypertens. 24:143–150. DOI: 10.1097/01.hjh.0000198024.91976.c2. PMID: 16331112. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=29144476760&origin=inward.35. Brotman DJ, Golden SH, Wittstein IS. 2007; The cardiovascular toll of stress. Lancet. 370:1089–1100. Erratum in: Lancet. 2007;370:1828. DOI: 10.1016/S0140-6736(07)61305-1. PMID: 17822755. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34548670283&origin=inward.36. Liao D, Sloan RP, Cascio WE, Folsom AR, Liese AD, Evans GW, Cai J, Sharrett AR. 1998; Multiple metabolic syndrome is associated with lower heart rate variability. The Atherosclerosis Risk in Communities Study. Diabetes Care. 21:2116–2122. DOI: 10.2337/diacare.21.12.2116. PMID: 9839103. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031737372&origin=inward.37. Min KB, Min JY, Paek D, Cho SI. 2008; The impact of the components of metabolic syndrome on heart rate variability: using the NCEP-ATP III and IDF definitions. Pacing Clin Electrophysiol. 31:584–591. DOI: 10.1111/j.1540-8159.2008.01045.x. PMID: 18439173. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=42649116100&origin=inward.38. Koskinen T, Kähönen M, Jula A, Mattsson N, Laitinen T, Keltikangas-Järvinen L, Viikari J, Välimäki I, Rönnemaa T, Raitakari OT. 2009; Metabolic syndrome and short-term heart rate variability in young adults. The cardiovascular risk in young Finns study. Diabet Med. 26:354–361. DOI: 10.1111/j.1464-5491.2009.02686.x. PMID: 19388964. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=64249094842&origin=inward.39. Keerthi GS, Pal P, Pal GK, Sahoo JP, idhar MG Sr, Balachander J. 2016; Attenuated baroreflex sensitivity in normotensive prediabetes and diabetes in Indian adults. Endocr Res. 41:89–97. DOI: 10.3109/07435800.2015.1076454. PMID: 26513377. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84945324501&origin=inward.40. Li Z, Tang ZH, Zeng F, Zhou L. 2013; Associations between the severity of metabolic syndrome and cardiovascular autonomic function in a Chinese population. J Endocrinol Invest. 36:993–999. DOI: 10.3275/9005. PMID: 23770583. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84886404812&origin=inward.41. Rasic-Milutinovic ZR, Milicevic DR, Milovanovic BD, Perunicic-Pekovic GB, Pencic BD. 2010; Do components of metabolic syndrome contribute to cardiac autonomic neuropathy in non-diabetic patients? Saudi Med J. 31:650–657. PMID: 20563363. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77958048542&origin=inward.42. Balcıoğlu AS, Akıncı S, Çiçek D, Çoner A, Bal UA, Müderrisoğlu İH. 2016; Cardiac autonomic nervous dysfunction detected by both heart rate variability and heart rate turbulence in prediabetic patients with isolated impaired fasting glucose. Anatol J Cardiol. 16:762–769. DOI: 10.14744/AnatolJCardiol.2015.6654. PMCID: PMC5324936. PMID: 27025199. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84994324029&origin=inward.43. Carnagarin R, Matthews VB, Herat LY, Ho JK, Schlaich MP. 2018; Autonomic regulation of glucose homeostasis: a specific role for sympathetic nervous system activation. Curr Diab Rep. 18:107. DOI: 10.1007/s11892-018-1069-2. PMID: 30232652. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85053450279&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association between Open Angle Glaucoma and Metabolic Syndrome in Postmenopausal Women
- Relationship between Metabolic Syndrome and Triglyceride/High-density Lipoprotein Cholesterol Ratio
- Letter: Cut-off Values and Clinical Utility of Surrogate Markers for Insulin Resistance and Beta-Cell Function to Identify Metabolic Syndrome and Its Components among Southern Indian Adults
- Hypothyroidism and Metabolic Syndrome
- Response: Cut-off Values and Clinical Utility of Surrogate Markers for Insulin Resistance and Beta-Cell Function to Identify Metabolic Syndrome and Its Components among Southern Indian Adults