Korean J healthc assoc Infect Control Prev.
2022 Dec;27(2):96-103. 10.14192/kjicp.2022.27.2.96.
Current Status and Prospects of the National Antimicrobial Resistance Surveillance System, Kor-GLASS
- Affiliations
-
- 1Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, Chonnam National University Medical School, Gwangju, Korea
- KMID: 2537070
- DOI: http://doi.org/10.14192/kjicp.2022.27.2.96
Abstract
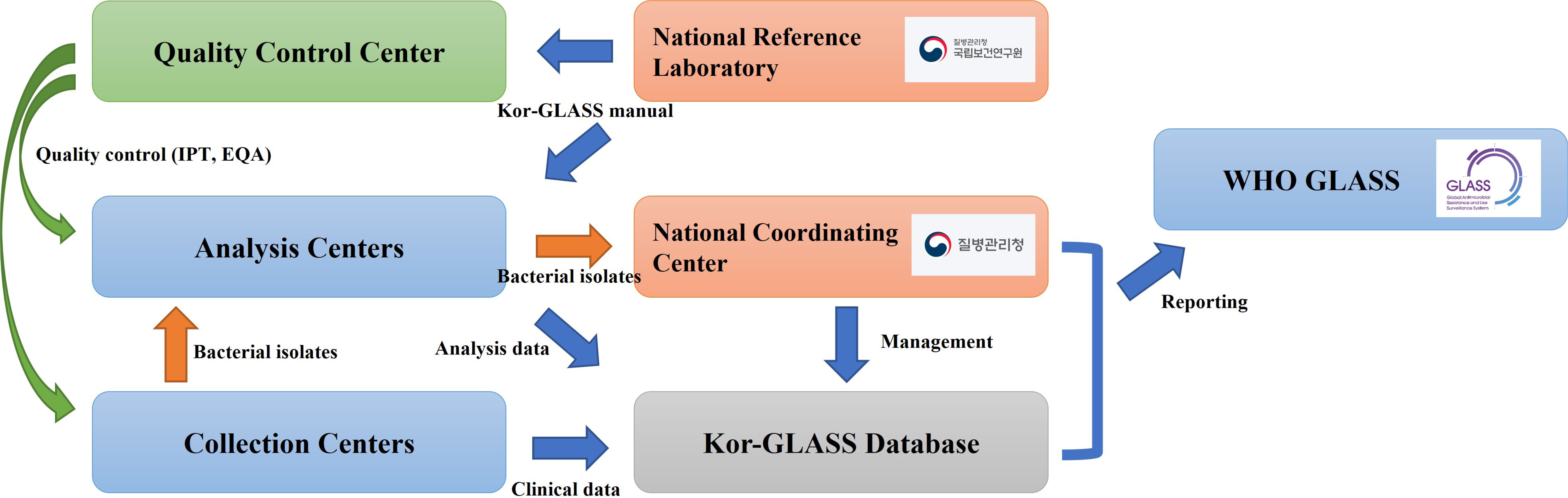
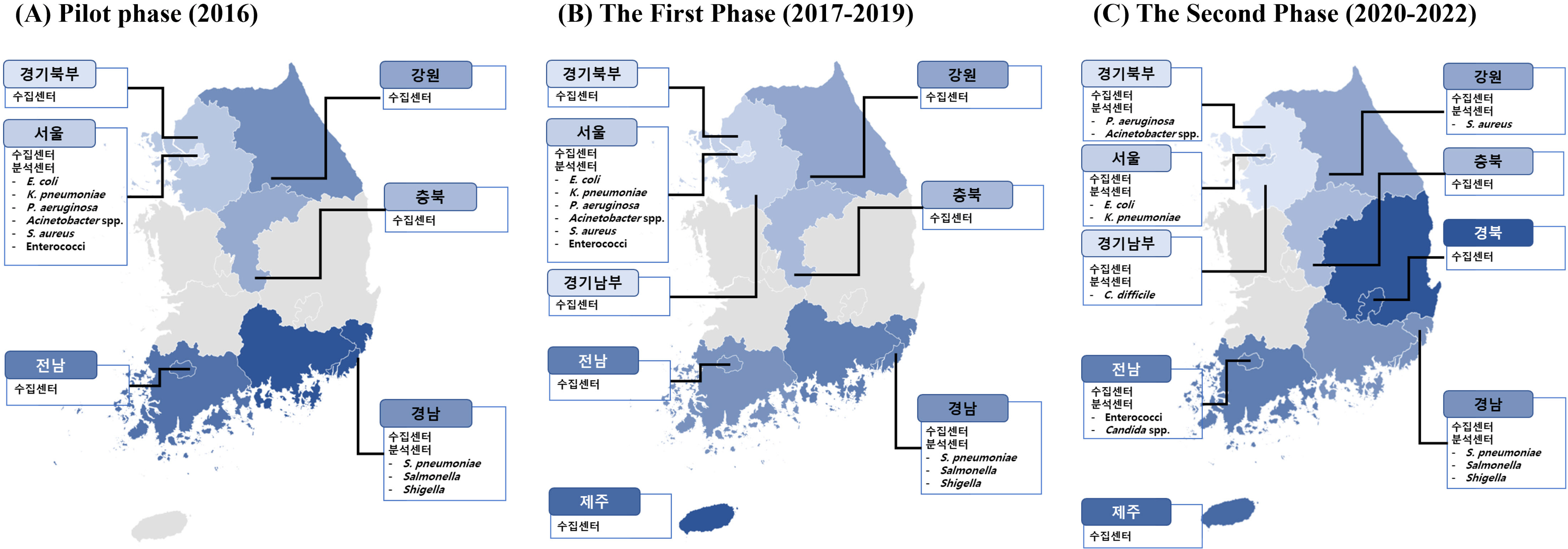
- Antimicrobial-resistant bacteria have been increasingly reported worldwide, and surveillance plays an important role in preventing the further dissemination of these organisms. The World Health Organization suggested the Global Antimicrobial Resistance Surveillance System (GLASS) as a part of a global action plan in 2015. The purpose of GLASS was to establish a worldwide surveillance system to collect standardized, comparable, and validated antimicrobial resistance (AMR) data, which would enable the comparison of AMR data by country. The Korean government established a new AMR surveillance system, namely Kor-GLASS, based on the GLASS platform in 2016. Kor-GLASS has several advantages over previous AMR systems: 1) standardized AMR data based on a strain-collection system, 2) characterization of multidrug-resistant clones by molecular epidemiologic evaluation, 3) collection of the clinical information related to bacterial isolates, and 4) an independent quality control center and the Kor-GLASS database. Based on a successful pilot program, the first phase of Kor-GLASS operated from 2017 to 2019, and the second phase (2020-2022) of the system is now underway. Kor-GLASS provides comprehensive AMR surveillance data, and the trends of AMR epidemiology are determined by molecular characterization. Furthermore, it enables a global comparison of AMR with that in other GLASS-enrolled countries owing to the harmonized platform. Kor-GLASS should be further improved to provide sustainable and reliable AMR data by establishing additional collecting centers for representativeness, covering community infection-associated AMR, and investigating emerging AMR.
Keyword
Figure
Cited by 1 articles
-
Korean National Healthcare-associated Infections Surveillance System, Intensive Care Unit Module Report: Summary of Data from July 2020 through June 2021
Eun Jin Kim, Yee Gyung Kwak, Sun Hee Kwak, Su Hui Ko, Oh Mee Kweon, Eu Suk Kim, Jin Hwa Kim, Tae Hyong Kim, Taek Soo Kim, Hee-Won Moon, Sun Hee Park, Jin Young Ahn, So-Yeon Yoo, Hyeon Mi Yoo, Sang-Oh Lee, Yu-Mi Lee, Nan-Hyoung Cho, Young Hwa Choi, Pyoeng Gyun Choe, Ki Ho Hong, Mi Suk Lee
Korean J Healthc Assoc Infect Control Prev. 2023;28(1):64-77. doi: 10.14192/kjicp.2023.28.1.64.
Reference
-
1. Maragakis LL, Perencevich EN, Cosgrove SE. 2008; Clinical and economic burden of antimicrobial resistance. Expert Rev Anti Infect Ther. 6:751–63. DOI: 10.1586/14787210.6.5.751. PMID: 18847410.
Article2. Mehl A, Åsvold BO, Lydersen S, Paulsen J, Solligård E, Damås JK, et al. 2017; Burden of bloodstream infection in an area of Mid-Norway 2002-2013: a prospective population-based observational study. BMC Infect Dis. 17:205. DOI: 10.1186/s12879-017-2291-2. PMID: 28284196. PMCID: PMC5346205.
Article3. Giani T, Arena F, Pollini S, Di Pilato V, D'Andrea MM, Henrici De Angelis L, et al. 2018; Italian nationwide survey on Pseudomonas aeruginosa from invasive infections: activity of ceftolozane/tazobactam and comparators, and molecular epidemiology of carbapenemase producers. J Antimicrob Chemother. 73:664–71. DOI: 10.1093/jac/dkx453. PMID: 29216350.
Article4. Chong Y, Lee K, Park YJ, Jeon DS, Lee MH, Kim MY, et al. 1998; Korean nationwide surveillance of antimicrobial resistance of bacteria in 1997. Yonsei Med J. 39:569–77. DOI: 10.3349/ymj.1998.39.6.569. PMID: 10097685.
Article5. Lee K, Chang CL, Lee NY, Kim HS, Hong KS, Cho HC. 2000; Korean nationwide surveillance of antimicrobial resistance of bacteria in 1998. Yonsei Med J. 41:497–506. DOI: 10.3349/ymj.2000.41.4.497. PMID: 10992812.
Article6. Yong D, Shin HB, Kim YK, Cho J, Lee WG, Ha GY, et al. 2014; Increase in the prevalence of carbapenem-resistant Acinetobacter isolates and ampicillin-resistant non-typhoidal Salmonella species in Korea: a KONSAR study conducted in 2011. Infect Chemother. 46:84–93. DOI: 10.3947/ic.2014.46.2.84. PMID: 25024870. PMCID: PMC4091365.
Article7. Kim D, Ahn JY, Lee CH, Jang SJ, Lee H, Yong D, et al. 2017; Increasing resistance to extended-spectrum cephalosporins, fluoroquinolone, and carbapenem in gram-negative bacilli and the emergence of carbapenem non-susceptibility in Klebsiella pneumoniae: analysis of Korean Antimicrobial Resistance Monitoring System (KARMS) data from 2013 to 2015. Ann Lab Med. 37:231–9. DOI: 10.3343/alm.2017.37.3.231. PMID: 28224769. PMCID: PMC5339095.
Article8. World Health Organization. National antimicrobial resistance surveillance systems and participation in the Global Antimicrobial Resistance Surveillance System (GLASS): a guide to planning, implementation, and monitoring and evaluation. https://apps.who.int/iris/handle/10665/251554. Updated on 2016.9. Lee H, Yoon EJ, Kim D, Jeong SH, Shin JH, Shin JH, et al. 2018; Establishment of the South Korean national antimicrobial resistance surveillance system, Kor-GLASS, in 2016. Euro Surveill. 23:1700734. DOI: 10.2807/1560-7917.ES.2018.23.42.1700734. PMID: 30352643. PMCID: PMC6199867.
Article10. Lee H, Yoon EJ, Kim D, Jeong SH, Won EJ, Shin JH, et al. 2018; Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: first one-year report from Kor-GLASS. Euro Surveill. 23:1800047. DOI: 10.2807/1560-7917.ES.2018.23.42.1800047. PMID: 30352640. PMCID: PMC6199864.
Article11. Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing. CLSI document M100S. 26th ed. CLSI;Wayne: p. 252. DOI: 10.1016/s0196-4399(01)88009-0.12. European Committee on Antimicrobial Susceptibility Testing. Clinical breakpoints - bacteria (v 6.0). AST clinical breakpoint. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf. Updated on 1 January 2016.13. European Committee on Antimicrobial Susceptibility Testing. EUCAST guideline for the detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. https://www.eucast.org/resistance_mechanisms/. Updated on 11 July 2017.14. Kim D, Yoon EJ, Hong JS, Choi MH, Kim HS, Kim YR, et al. 2022; Major bloodstream infection-causing bacterial pathogens and their antimicrobial resistance in South Korea, 2017-2019: phase I report from Kor-GLASS. Front Microbiol. 12:799084. DOI: 10.3389/fmicb.2021.799084. PMID: 35069503. PMCID: PMC8770956.
Article15. Liu C, Yoon EJ, Kim D, Shin JH, Shin JH, Shin KS, et al. 2019; Antimicrobial resistance in South Korea: a report from the Korean global antimicrobial resistance surveillance system (Kor-GLASS) for 2017. J Infect Chemother. 25:845–59. DOI: 10.1016/j.jiac.2019.06.010. PMID: 31311694.
Article16. World Health Organization. The global antimicrobial resistance surveillance system (GLASS) report: early implementation 2017-2018. https://www.who.int/publications/i/item/9789241515061. Updated on 1 January 2019.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of multidrug-resistant bacteria
- Current aspects and prospects of glass ionomer cements for clinical dentistry
- Detection of Tigecycline Resistance in Acinetobacter baumannii: The Discrepancy between the Minimal Inhibitory Concentration Method and the Disk Diffusion Test
- Four genotypes of carbapenem-resistant Acinetobacter baumannii strains lacking OXA-23 production in Korea
- The crisis of antimicrobial resistance: current status and future strategies