Nutr Res Pract.
2022 Dec;16(6):729-744. 10.4162/nrp.2022.16.6.729.
L-Methionine inhibits 4-hydroxy-2-nonenal accumulation and suppresses inflammation in growing rats
- Affiliations
-
- 1Department of Food Science and Engineering, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin 150001, China
- KMID: 2536507
- DOI: http://doi.org/10.4162/nrp.2022.16.6.729
Abstract
- BACKGROUND/OBJECTIVES
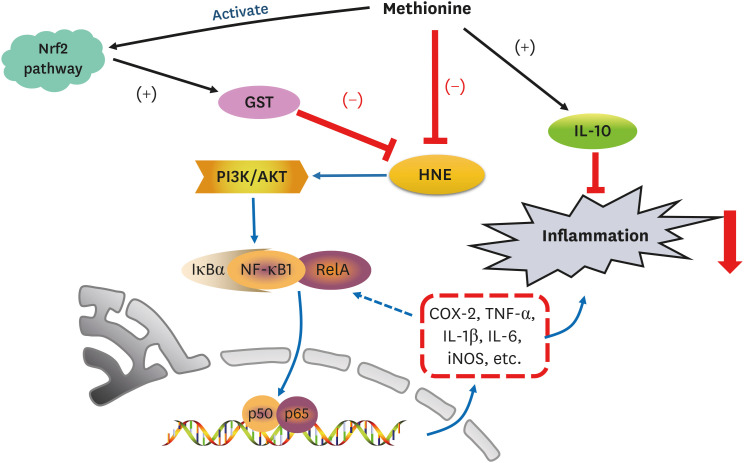
4-Hydroxy-2-nonenal (HNE) is a biomarker for oxidative stress to induce inflammation. Methionine is an essential sulfur-containing amino acid with antioxidative activity. On the other hand, the evidence on whether and how methionine can depress HNE-derived inflammation is lacking. In particular, the link between the regulation of the nuclear factor-κB (NF-κB) signaling pathway and methionine intake is unclear. This study examined the link between depression from HNE accumulation and the antiinflammatory function of L-methionine in rats.
MATERIALS/METHODS
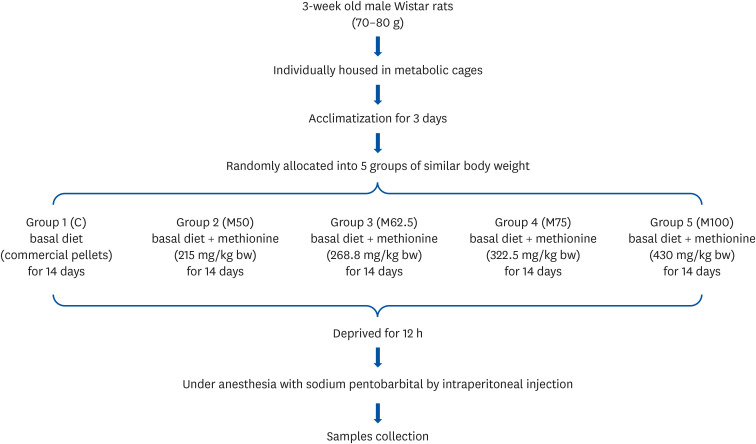
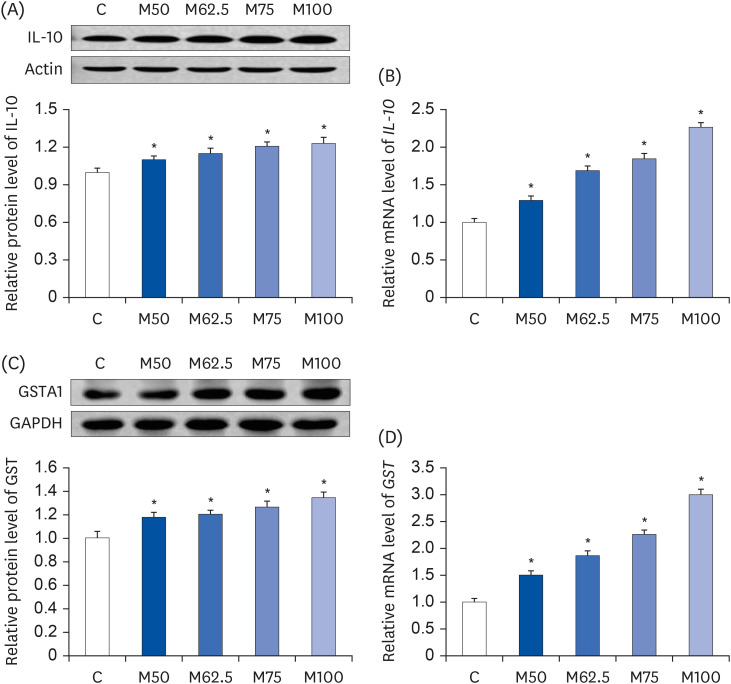
Male Wistar rats (3-week-old, weighing 70–80 g) were administered different levels of L-methionine orally at 215.0, 268.8, 322.5, and 430.0 mg/kg body weight for two weeks. The control group was fed commercial pellets. The hepatic HNE contents and the protein expression and mRNA levels of the inflammatory mediators were measured. The interleukin-10 (IL-10) and glutathione S-transferase (GST) levels were also estimated.
RESULTS
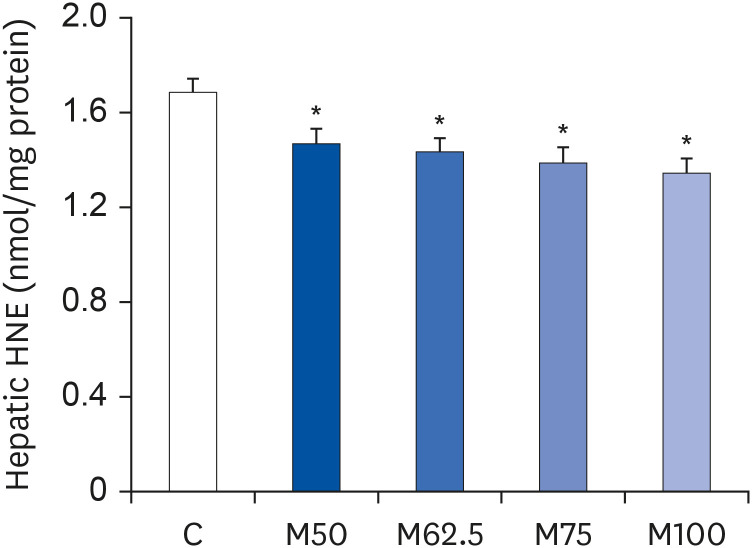
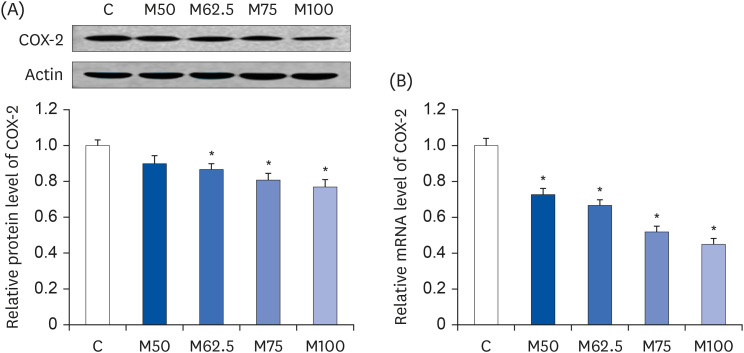
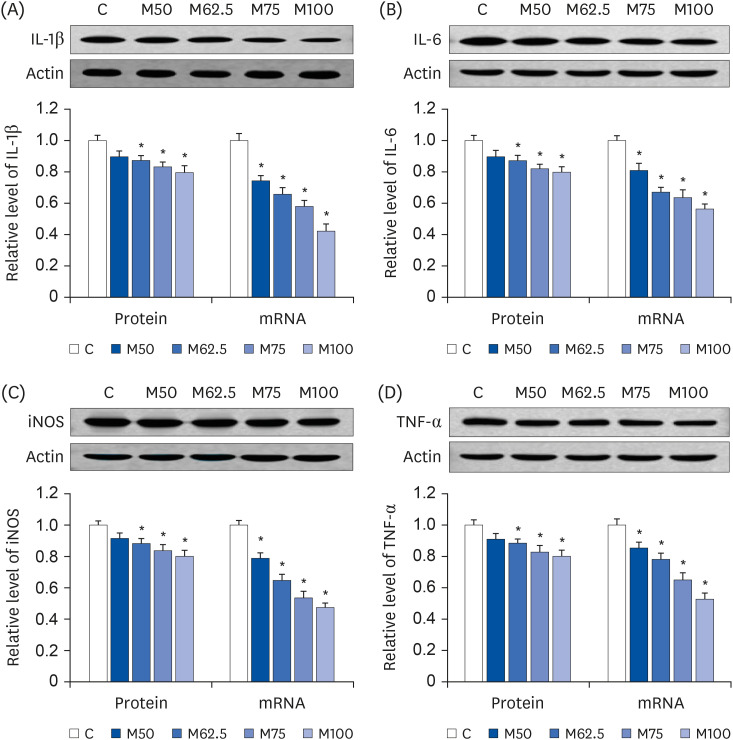
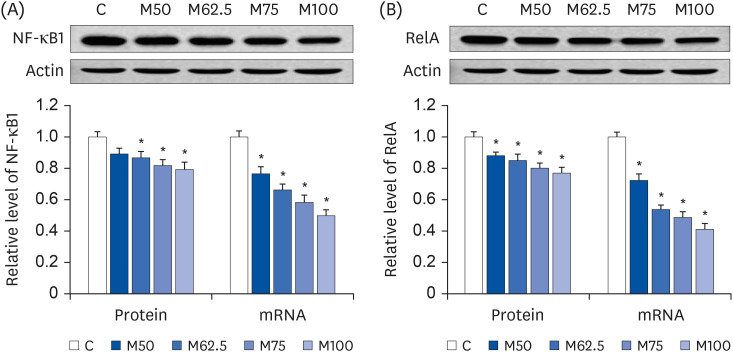
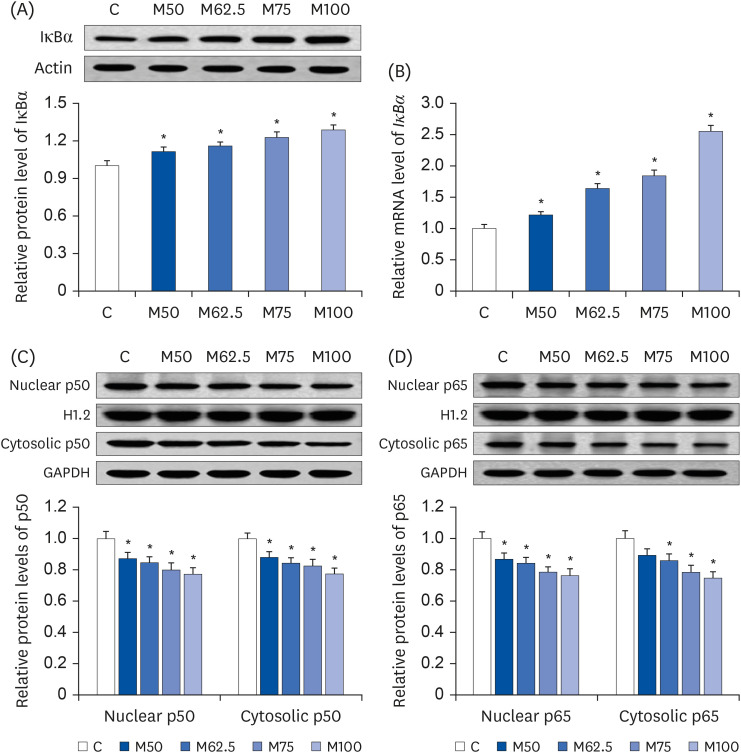
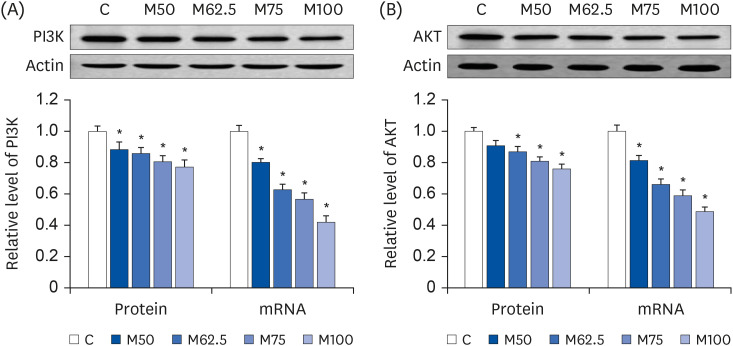
Compared to the control group, hepatic HNE levels were reduced significantly in all groups fed L-methionine, which were attributed to the stimulation of GST by L-methionine. With decreasing HNE levels, L-methionine inhibited the activation of NF-κB by up-regulating inhibitory κBα and depressing phosphoinositide 3 kinase/protein kinase B. The mRNA levels of the inflammatory mediators (cyclooxygenase-2, interleukin-1β, interleukin-6, inducible nitric oxide synthase, tumor necrotic factor alpha) were decreased significantly by L-methionine. In contrast, the protein expression of these inflammatory mediators was effectively down regulated by L-methionine. The anti-inflammatory action of L-methionine was also reflected by the up-regulation of IL-10.
CONCLUSIONS
This study revealed a link between the inhibition of HNE accumulation and the depression of inflammation in growing rats, which was attributed to L-methionine availability. The anti-inflammatory mechanism exerted by L-methionine was to inhibit NF-κB activation and to up-regulate GST.
Keyword
Figure
Reference
-
1. Park CM, Song YS. Luteolin and luteolin-7-O-glucoside protect against acute liver injury through regulation of inflammatory mediators and antioxidative enzymes in GalN/LPS-induced hepatitic ICR mice. Nutr Res Pract. 2019; 13:473–479. PMID: 31814922.
Article2. Kim JN, Han SN, Ha TJ, Kim HK. Black soybean anthocyanins attenuate inflammatory responses by suppressing reactive oxygen species production and mitogen activated protein kinases signaling in lipopolysaccharide-stimulated macrophages. Nutr Res Pract. 2017; 11:357–364. PMID: 28989571.
Article3. Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011; 13:11–22. PMID: 21195345.
Article4. Romano A, Serviddio G, Calcagnini S, Villani R, Giudetti AM, Cassano T, Gaetani S. Linking lipid peroxidation and neuropsychiatric disorders: focus on 4-hydroxy-2-nonenal. Free Radic Biol Med. 2017; 111:281–293. PMID: 28063940.
Article5. Uchida K. HNE as an inducer of COX-2. Free Radic Biol Med. 2017; 111:169–172. PMID: 28192229.
Article6. Zarkovic K, Jakovcevic A, Zarkovic N. Contribution of the HNE-immunohistochemistry to modern pathological concepts of major human diseases. Free Radic Biol Med. 2017; 111:110–126. PMID: 27993730.
Article7. Zhang H, Forman HJ. 4-hydroxynonenal-mediated signaling and aging. Free Radic Biol Med. 2017; 111:219–225. PMID: 27876535.
Article8. Nègre-Salvayre A, Garoby-Salom S, Swiader A, Rouahi M, Pucelle M, Salvayre R. Proatherogenic effects of 4-hydroxynonenal. Free Radic Biol Med. 2017; 111:127–139. PMID: 28040472.
Article9. Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008; 7:83–105. PMID: 17964225.
Article10. Lee A, Gu H, Gwon MH, Yun JM. Hesperetin suppresses LPS/high glucose-induced inflammatory responses via TLR/MyD88/NF-κB signaling pathways in THP-1 cells. Nutr Res Pract. 2021; 15:591–603. PMID: 34603607.
Article11. Lee H, Park C, Kwon DH, Hwangbo H, Kim SY, Kim MY, Ji SY, Kim DH, Jeong JW, Kim GY, et al. Schisandrae Fructus ethanol extract attenuates particulate matter 2.5-induced inflammatory and oxidative responses by blocking the activation of the ROS-dependent NF-κB signaling pathway. Nutr Res Pract. 2021; 15:686–702. PMID: 34858548.
Article12. Limtrakul P, Yodkeeree S, Pitchakarn P, Punfa W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages. Nutr Res Pract. 2016; 10:251–258. PMID: 27247720.
Article13. Sung J, Sung M, Kim Y, Ham H, Jeong HS, Lee J. Anti-inflammatory effect of methanol extract from Erigeron Canadensis L. may be involved with upregulation of heme oxygenase-1 expression and suppression of NFκB and MAPKs activation in macrophages. Nutr Res Pract. 2014; 8:352–359. PMID: 25110553.
Article14. Lee JE, Cho SM, Park E, Lee SM, Kim Y, Auh JH, Choi HK, Lim S, Lee SC, Kim JH. Anti-inflammatory effects of Rubus coreanus Miquel through inhibition of NF-κB and MAP Kinase. Nutr Res Pract. 2014; 8:501–508. PMID: 25324928.
Article15. Kim SY, Kim H, Min H. Effects of excessive dietary methionine on oxidative stress and dyslipidemia in chronic ethanol-treated rats. Nutr Res Pract. 2015; 9:144–149. PMID: 25861420.
Article16. Yang L, Kadowaki M. Addition of methionine to rice protein affects hepatic cholesterol output inducing hypocholesterolemia in rats fed cholesterol-free diets. J Med Food. 2011; 14:445–453. PMID: 21434776.
Article17. Wang Z, Liang M, Li H, Cai L, He H, Wu Q, Yang L. L-Methionine activates Nrf2-ARE pathway to induce endogenous antioxidant activity for depressing ROS-derived oxidative stress in growing rats. J Sci Food Agric. 2019; 99:4849–4862. PMID: 31001831.
Article18. Wang Z, Cai L, Li H, Liang M, Zhang Y, Wu Q, Yang L. Rice protein stimulates endogenous antioxidant response attributed to methionine availability in growing rats. J Food Biochem. 2020; 44:e13180. PMID: 32163604.
Article19. Oda H. Functions of sulfur-containing amino acids in lipid metabolism. J Nutr. 2006; 136(Suppl):1666S–9S. PMID: 16702337.
Article20. Martínez Y, Li X, Liu G, Bin P, Yan W, Más D, Valdivié M, Hu CA, Ren W, Yin Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids. 2017; 49:2091–2098. PMID: 28929442.
Article21. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951. PMID: 8229312.
Article22. Li H, Cai L, Liang M, Wang Z, Zhang Y, Wu Q, Yang L. Methionine augments endogenous antioxidant capacity of rice protein through stimulating methionine sulfoxide reductase antioxidant system and activating Nrf2-ARE pathway in growing and adult rats. Eur Food Res Technol. 2020; 246:1051–1063.
Article23. Liang M, Wang Z, Li H, Cai L, Pan J, He H, Wu Q, Tang Y, Ma J, Yang L. L-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem Toxicol. 2018; 115:315–328. PMID: 29577948.
Article24. Wang Z, Liang M, Li H, Cai L, Yang L. Rice protein exerts anti-inflammatory effect in growing and adult rats via suppressing NF-κB pathway. Int J Mol Sci. 2019; 20:6164. PMID: 31817701.
Article25. Natarajan K, Abraham P, Kota R, Isaac B. NF-κB-iNOS-COX2-TNF α inflammatory signaling pathway plays an important role in methotrexate induced small intestinal injury in rats. Food Chem Toxicol. 2018; 118:766–783. PMID: 29935243.
Article26. Lu SC, Mato JM, Espinosa-Diez C, Lamas S. MicroRNA-mediated regulation of glutathione and methionine metabolism and its relevance for liver disease. Free Radic Biol Med. 2016; 100:66–72. PMID: 27033954.
Article27. Métayer S, Seiliez I, Collin A, Duchêne S, Mercier Y, Geraert PA, Tesseraud S. Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J Nutr Biochem. 2008; 19:207–215. PMID: 17707628.
Article28. Li H, Wang Z, Liang M, Cai L, Yang L. Methionine augments antioxidant activity of rice protein during gastrointestinal digestion. Int J Mol Sci. 2019; 20:868. PMID: 30781587.
Article29. Di Domenico F, Tramutola A, Butterfield DA. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of Alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic Biol Med. 2017; 111:253–261. PMID: 27789292.
Article30. Mol M, Regazzoni L, Altomare A, Degani G, Carini M, Vistoli G, Aldini G. Enzymatic and non-enzymatic detoxification of 4-hydroxynonenal: methodological aspects and biological consequences. Free Radic Biol Med. 2017; 111:328–344. PMID: 28161307.
Article31. Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009; 30:42–59. PMID: 18601945.
Article32. Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013; 1830:3143–3153. PMID: 22995213.
Article33. Awasthi YC, Ramana KV, Chaudhary P, Srivastava SK, Awasthi S. Regulatory roles of glutathione-S-transferases and 4-hydroxynonenal in stress-mediated signaling and toxicity. Free Radic Biol Med. 2017; 111:235–243. PMID: 27794453.
Article34. Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, Awasthi S. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med. 2004; 37:607–619. PMID: 15288119.
Article35. Kriete A, Mayo KL. Atypical pathways of NF-kappaB activation and aging. Exp Gerontol. 2009; 44:250–255. PMID: 19174186.36. Kuan YH, Huang FM, Li YC, Chang YC. Proinflammatory activation of macrophages by bisphenol A-glycidyl-methacrylate involved NFκB activation via PI3K/Akt pathway. Food Chem Toxicol. 2012; 50:4003–4009. PMID: 22939937.
Article37. Madrid LV, Mayo MW, Reuther JY, Baldwin AS Jr. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-κ B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001; 276:18934–18940. PMID: 11259436.
Article38. Park CM, Cho CW, Song YS. TOP 1 and 2, polysaccharides from Taraxacum officinale, inhibit NFκB-mediated inflammation and accelerate Nrf2-induced antioxidative potential through the modulation of PI3K-Akt signaling pathway in RAW 264.7 cells. Food Chem Toxicol. 2014; 66:56–64. PMID: 24447978.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Resveratrol Supplementation on Oxidative Damage and Lipid Peroxidation Induced by Strenuous Exercise in Rats
- BCG infection during pre-sensitization or even post-sensitization inhibits airway sensitivity in an animal model of allergic asthma
- Studies on preparation of 99mTc complexes of methionine isomers
- The Effect of Combined Treatment of Cadmium and Methionine on the Accumulation of Cadmium in Liver and Kidney and the Activation of Alkaline Phosphatase in Blood of Mice
- 4-hydroxy-2(E)-Nonenal facilitates NMDA-Induced Neurotoxicity via Triggering Mitochondrial Permeability Transition Pore Opening and Mitochondrial Calcium Overload