Nutr Res Pract.
2022 Dec;16(6):685-699. 10.4162/nrp.2022.16.6.685.
Immunostimulatory activity of hydrolyzed and fermented Platycodon grandiflorum extract occurs via the MAPK and NF-κB signaling pathway in RAW 264.7 cells
- Affiliations
-
- 1Regional Strategic Industry Innovation Center, Hallym University, Chuncheon 24252, Korea
- 2Department of Food Science & Nutrition, Dongseo University, Busan 47011, Korea
- 3R&D Center, World Food Services Co. Ltd., Gangneung 25451, Korea
- KMID: 2536504
- DOI: http://doi.org/10.4162/nrp.2022.16.6.685
Abstract
- BACKGROUND/OBJECTIVES
Platycodon grandiflorum (PG) has long been known as a medicinal herb effective in various diseases, including bronchitis and asthma, but is still more widely used for food. Fermentation methods are being applied to increase the pharmacological composition of PG extracts and commercialize them with high added value. This study examines the hydrolyzed and fermented PG extract (HFPGE) fermented with Lactobacillus casei in RAW 264.7 cells, and investigates the effect of amplifying the immune and the probable molecular mechanism.
MATERIALS/METHODS
HFPGE’s total phenolic, flavonoid, saponin, and platycodin D contents were analyzed by colorimetric analysis or high-performance liquid chromatography. Cell viability was measured by the MTT assay. Phagocytic activity was analyzed by a phagocytosis assay kit, nitric oxide (NO) production by a Griess reagent system, and cytokines by enzyme-linked immunosorbent assay kits. The mRNA expressions of inducible nitric oxide synthase (iNOS) and cytokines were analyzed by reverse transcription-polymerase chain reaction, whereas MAPK and nuclear factor (NF)-κB activation were analyzed by Western blots.
RESULTS
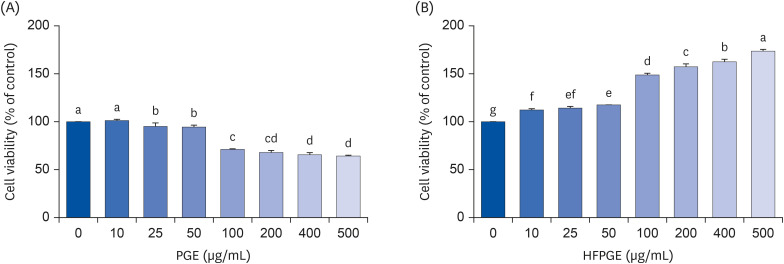
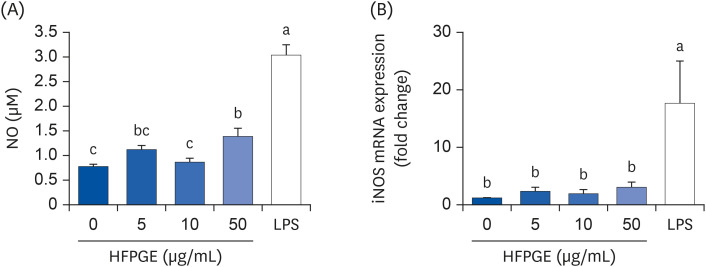
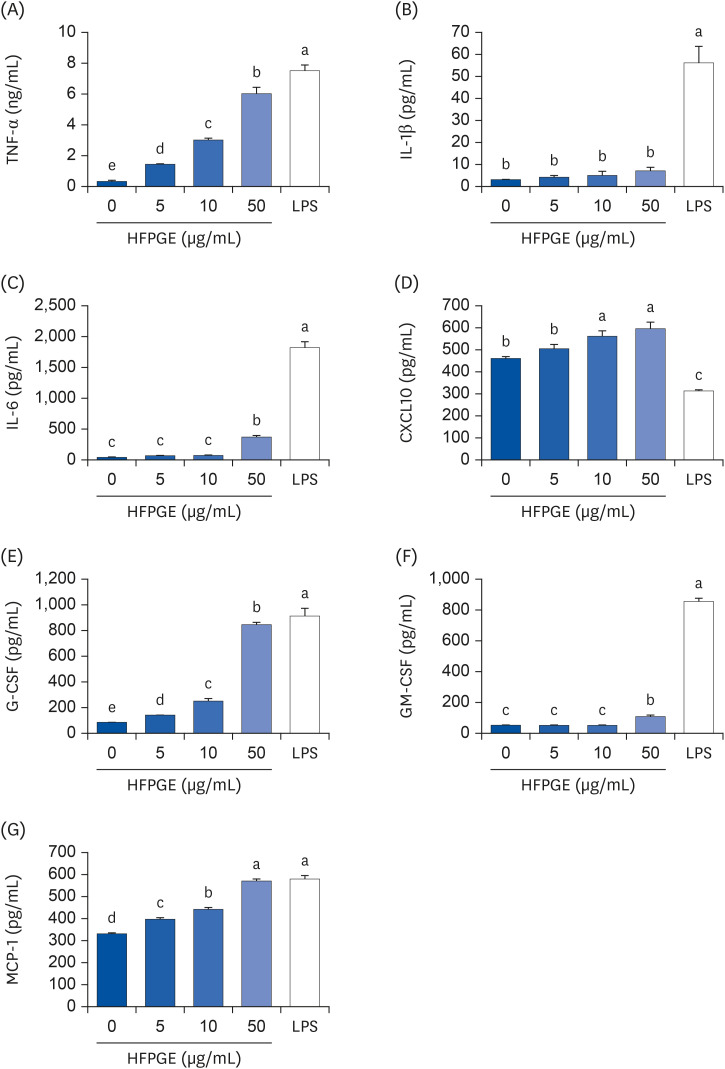
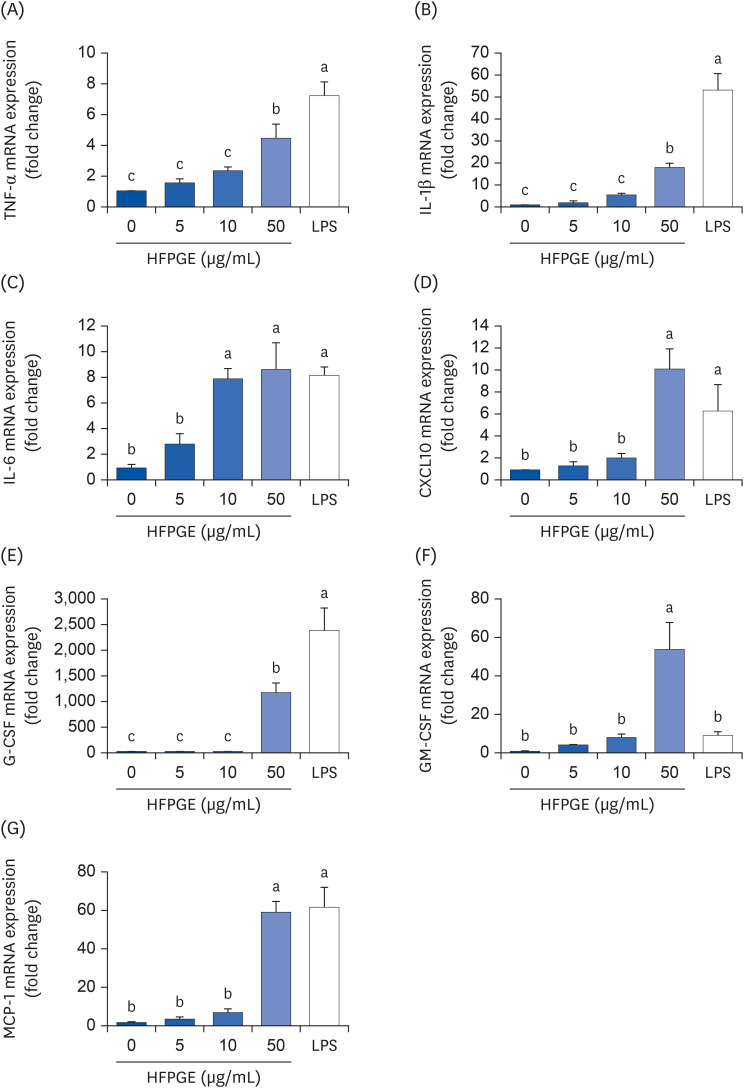
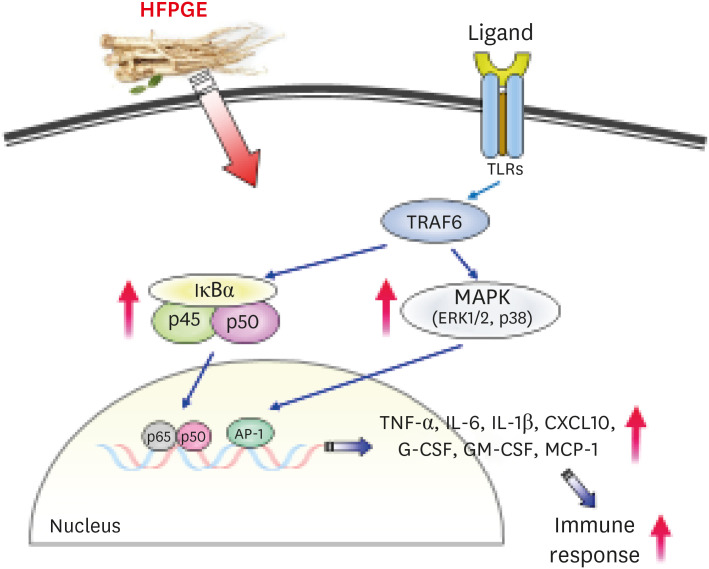
Compared to PGE, HFPGE was determined to contain 13.76 times and 6.69 times higher contents of crude saponin and platycodin D, respectively. HFPGE promoted cell proliferation and phagocytosis in RAW 264.7 cells and regulated the NO production and iNOS expression. Treatment with HFPGE also resulted in increased production of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, C-X-C motif chemokine ligand10, granulocytecolony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and monocyte chemoattractant protein-1, and the mRNA expressions of these cytokines. HFPGE also resulted in significantly increasing the phosphorylation of NF-κB p65, extracellular signalregulated kinase, and c-Jun N-terminal kinase.
CONCLUSIONS
Taken together, our results imply that fermentation and hydrolysis result in the extraction of more active ingredients of PG. Furthermore, we determined that HFPGE exerts immunostimulatory activity via the MAPK and NF-κB signaling pathways.
Keyword
Figure
Reference
-
1. Park HY, Shin JH, Boo HO, Gorinstein S, Ahn YG. Discrimination of Platycodon grandiflorum and Codonopsis lanceolata using gas chromatography-mass spectrometry-based metabolomics approach. Talanta. 2019; 192:486–491. PMID: 30348422.
Article2. Ji MY, Bo A, Yang M, Xu JF, Jiang LL, Zhou BC, Li MH. The pharmacological effects and health benefits of Platycodon grandiflorus—A medicine food homology species. Foods. 2020; 9:142. PMID: 32023858.
Article3. Zhang L, Wang Y, Yang D, Zhang C, Zhang N, Li M, Liu Y. Platycodon grandiflorus – An ethnopharmacological, phytochemical and pharmacological review. J Ethnopharmacol. 2015; 164:147–161. PMID: 25666431.
Article4. Nyakudya E, Jeong JH, Lee NK, Jeong YS. Platycosides from the roots of Platycodon grandiflorum and their health benefits. Prev Nutr Food Sci. 2014; 19:59–68. PMID: 25054103.
Article5. Li W, Tian YH, Liu Y, Wang Z, Tang S, Zhang J, Wang YP. Platycodin D exerts anti-tumor efficacy in H22 tumor-bearing mice via improving immune function and inducing apoptosis. J Toxicol Sci. 2016; 41:417–428. PMID: 27193733.
Article6. Huang YH, Jung DW, Lee OH, Kang IJ. Fermented Platycodon grandiflorum extract inhibits lipid accumulation in 3T3-L1 adipocytes and high-fat diet-induced obese mice. J Med Food. 2016; 19:1004–1014. PMID: 27792464.
Article7. Yoda K, Sun X, Kawase M, Kubota A, Miyazawa K, Harata G, Hosoda M, Hiramatsu M, He F, Zemel MB. A combination of probiotics and whey proteins enhances anti-obesity effects of calcium and dairy products during nutritional energy restriction in aP2-agouti transgenic mice. Br J Nutr. 2015; 113:1689–1696. PMID: 25871498.
Article8. Macfarlane GT, Steed H, Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol. 2008; 104:305–344. PMID: 18215222.
Article9. Lee S, Han EH, Lim MK, Lee SH, Yu HJ, Lim YH, Kang S. Fermented Platycodon grandiflorum extracts relieve airway inflammation and cough reflex sensitivity in vivo . J Med Food. 2020; 23:1060–1069. PMID: 32758004.
Article10. Singleton VL, Rossi TA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965; 16:144–158.11. Moreno MI, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000; 71:109–114. PMID: 10904153.
Article12. Chua LS, Lau CH, Chew CY, Dawood DA. Solvent fractionation and acetone precipitation for crude saponins from Eurycoma longifolia extract. Molecules. 2019; 24:1416. PMID: 30974893.
Article13. Kim EJ, Holthuizen PE, Park HS, Ha YL, Jung KC, Park JH. Trans-10,cis-12-conjugated linoleic acid inhibits Caco-2 colon cancer cell growth. Am J Physiol Gastrointest Liver Physiol. 2002; 283:G357–G367. PMID: 12121883.14. Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, Tyner AL, Park JH. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am J Physiol Gastrointest Liver Physiol. 2003; 284:G996–1005. PMID: 12571082.
Article15. Kim SR, Park EJ, Dusabimana T, Je J, Jeong K, Yun SP, Kim HJ, Cho KM, Kim H, Park SW. Platycodon grandiflorus fermented extracts attenuate endotoxin-induced acute liver injury in mice. Nutrients. 2020; 12:2802. PMID: 32933130.
Article16. Mercenier A, Pavan S, Pot B. Probiotics as biotherapeutic agents: present knowledge and future prospects. Curr Pharm Des. 2003; 9:175–191. PMID: 12570667.
Article17. Kim MS, Kim WG, Chung HS, Park BW, Ahn KS, Kim JJ, Bae H. Improvement of atopic dermatitis-like skin lesions by Platycodon grandiflorum fermented by Lactobacillus plantarum in NC/Nga mice. Biol Pharm Bull. 2012; 35:1222–1229. PMID: 22863917.
Article18. Li W, Zhang W, Xiang L, Wang Z, Zheng YN, Wang YP, Zhang J, Chen L, Platycoside N. Platycoside N: a new oleanane-type triterpenoid saponin from the roots of Platycodon grandiflorum . Molecules. 2010; 15:8702–8708. PMID: 21119565.
Article19. Jung JA, Noh JH, Jang MS, Gu EY, Cho MK, Lim KH, Park H, Back SM, Kim SP, Han KH. Safety evaluation of fermented Platycodon grandiflorus (Jacq.) A.DC. extract: genotoxicity, acute toxicity, and 13-week subchronic toxicity study in rats. J Ethnopharmacol. 2021; 275:114138. PMID: 33895248.20. Choi CY, Kim JY, Kim YS, Chung YC, Hahm KS, Jeong HG. Augmentation of macrophage functions by an aqueous extract isolated from Platycodon grandiflorum . Cancer Lett. 2001; 166:17–25. PMID: 11295282.
Article21. Yoon YD, Han SB, Kang JS, Lee CW, Park SK, Lee HS, Kang JS, Kim HM. Toll-like receptor 4-dependent activation of macrophages by polysaccharide isolated from the radix of Platycodon grandiflorum . Int Immunopharmacol. 2003; 3:1873–1882. PMID: 14636836.
Article22. Sanchez-Ramos J, Song S, Sava V, Catlow B, Lin X, Mori T, Cao C, Arendash GW. Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer’s mice. Neuroscience. 2009; 163:55–72. PMID: 19500657.
Article23. Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS Jr. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity. 2013; 39:697–710. PMID: 24138881.
Article24. Xia M, Sui Z. Recent developments in CCR2 antagonists. Expert Opin Ther Pat. 2009; 19:295–303. PMID: 19441905.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunomodulatory effects of fermented Platycodon grandiflorum extract through NF-κB signaling in RAW 264.7 cells
- Galangin Regulates Mucin 5AC Gene Expression via the Nuclear Factor-κB Inhibitor α/Nuclear Factor-κB p65 Pathway in Human Airway Epithelial Cells
- Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages
- Wnt-C59 inhibits proinflammatory cytokine expression by reducing the interaction between β-catenin and NF-κB in LPS-stimulated epithelial and macrophage cells
- Aromadendrin Inhibits Lipopolysaccharide-Induced Nuclear Translocation of NF-kappaB and Phosphorylation of JNK in RAW 264.7 Macrophage Cells