Ann Hepatobiliary Pancreat Surg.
2022 Nov;26(4):363-374. 10.14701/ahbps.22-023.
Neoadjuvant therapy impact in early pancreatic cancer: “bioborderline” vs. “non-bioborderline”
- Affiliations
-
- 1Transplant and Hepatobiliopancreatic Surgery Unit, Department of General and Digestive Surgery, Hospital General Universitario Gregorio Marañón, Complutense University of Madrid, Madrid, Spain
- 2Department of Medical Oncology, Hospital General Universitario Gregorio Marañón, Madrid, Spain
- KMID: 2536387
- DOI: http://doi.org/10.14701/ahbps.22-023
Abstract
- Backgrounds/Aims
To analyze the results of the neoadjuvant treatment of patients in our center with early pancreatic cancer.
Methods
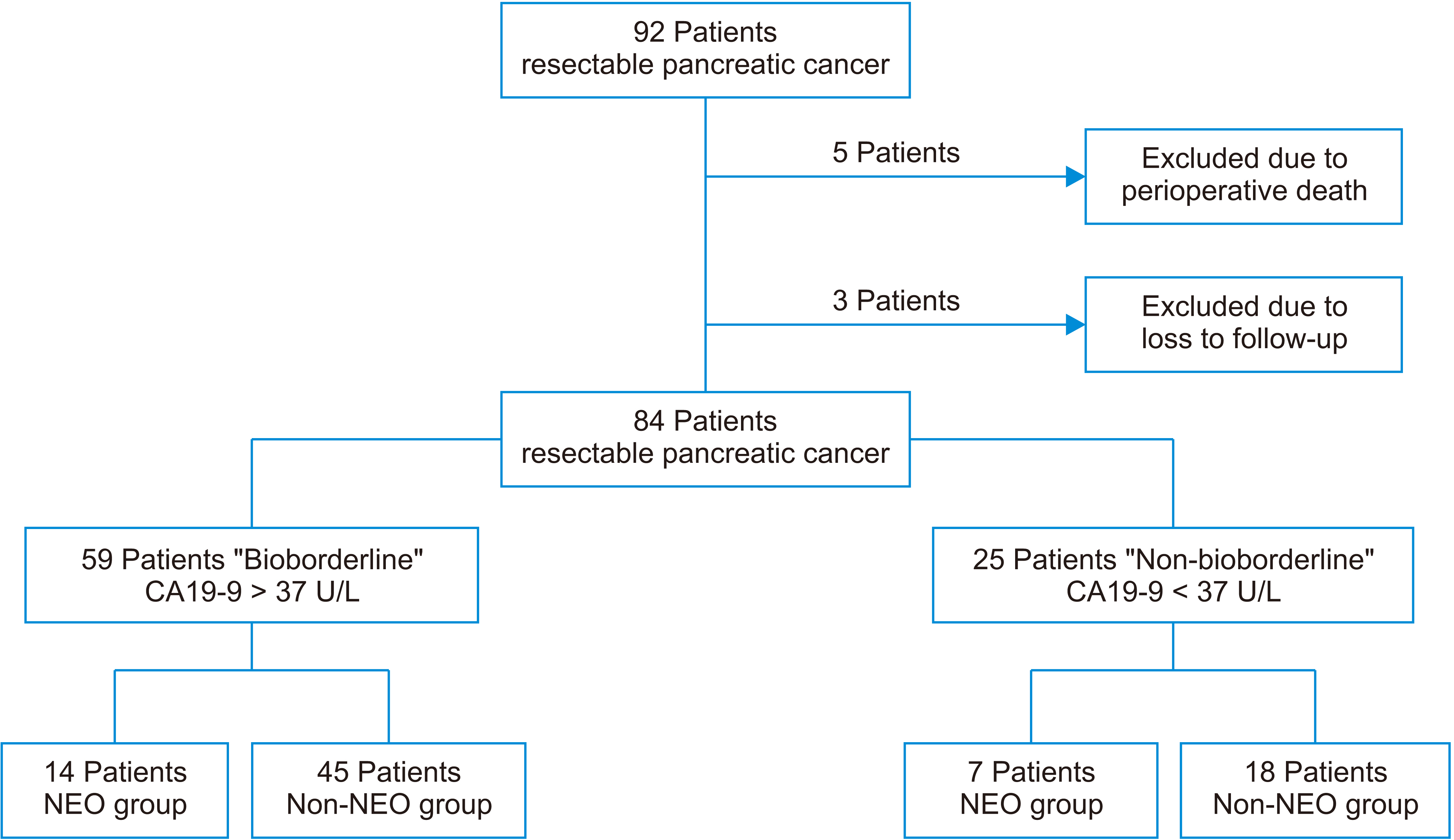
Eighty-four patients with early pancreatic cancer (I–II) were included, of which 59 were considered “bioborderline” (carbohydrate antigen [CA] 19-9 > 37 U/L), and 25 were considered “non-bioborderline” (CA19-9 < 37 U/L). The R0 resection rate, presence of negative nodes, survival, and recurrence rates were analyzed in two groups, the NEO group (neoadjuvant + surgery) and the nonNEO group (upfront surgery).
Results
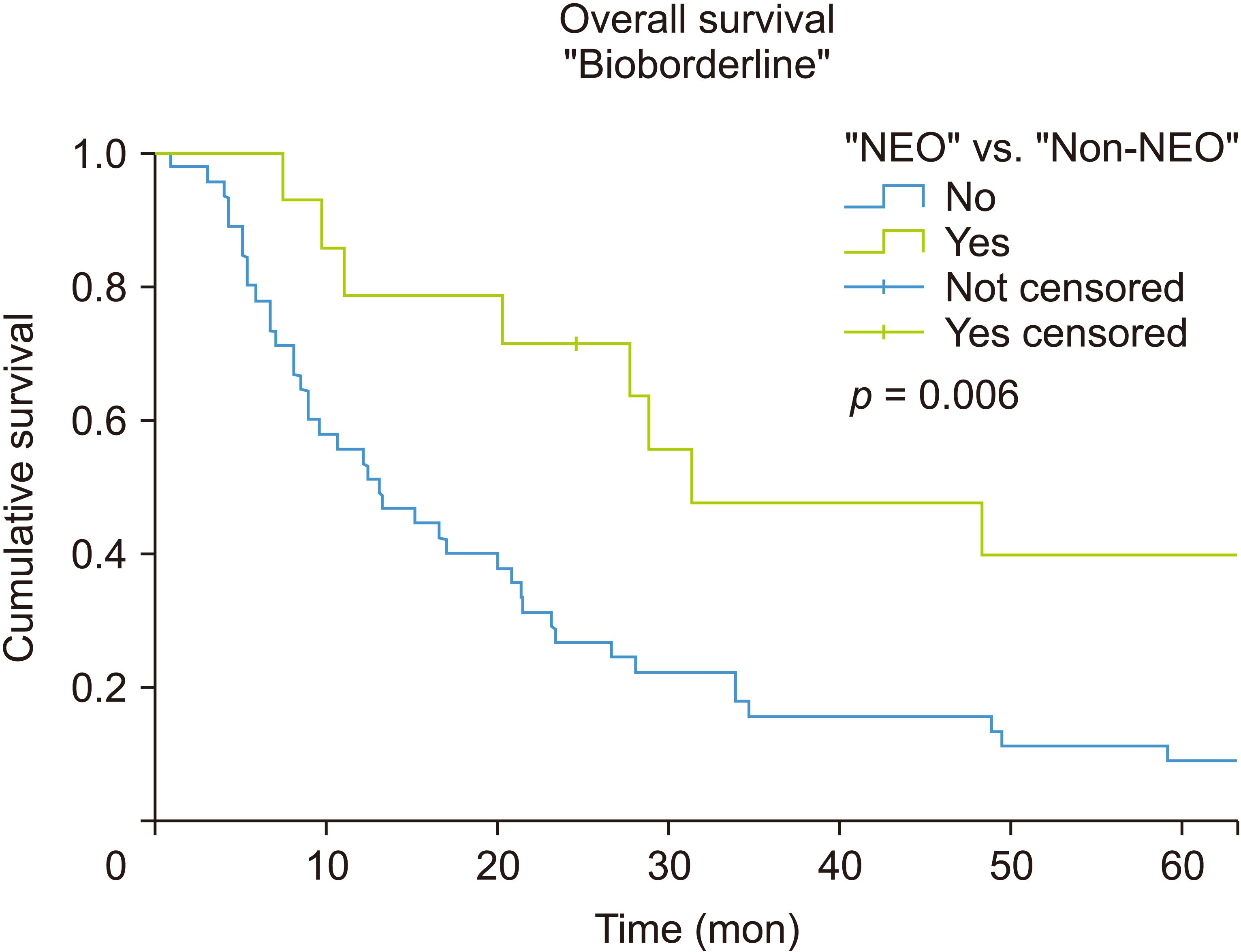
A 28.6% pathologic complete response was observed in the NEO group of the whole sample. The residual R0 was 85.7%, and nodes were negative in 78.6% of the patients in the NEO group of bioborderline patients. All non-bioborderline patients treated with neoadjuvant were R0, and no affected nodes were observed in any of them. The median overall survival (OS) in patients with elevated CA19-9 levels in the NEO group was 31.4 months vs. 13.1 months in the non-NEO (log-rank test p = 0.006), with a 62% relative reduction in the mortality rate (hazard ratio = 0.38, 95% confidence interval: 0.20–0.79; p = 0.008). The median OS in patients with normal CA19-9 levels in the NEO group was 65.9 months vs. 16.2 months in the non-NEO group, without statistically significant differences between the two but with a trend toward significance (log-rank test p = 0.08).
Conclusions
A neoadjuvant strategy seemed to improve local control and the survival of patients with early pancreatic cancer, both those with elevated CA19-9 and normal marker levels.
Figure
Reference
-
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. 2014; Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74:2913–2921. Erratum in: Cancer Res 2014;74:4006. DOI: 10.1158/0008-5472.CAN-14-0155. PMID: 24840647.2. Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. 2000; Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 4:567–579. DOI: 10.1016/S1091-255X(00)80105-5. PMID: 11307091.3. Griffin JF, Smalley SR, Jewell W, Paradelo JC, Reymond RD, Hassanein RE, et al. 1990; Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 66:56–61. DOI: 10.1002/1097-0142(19900701)66:1<56::AID-CNCR2820660112>3.0.CO;2-6. PMID: 2354408.4. Siegel RL, Miller KD, Jemal A. 2018; Cancer statistics, 2018. CA Cancer J Clin. 68:7–30. DOI: 10.3322/caac.21442. PMID: 29313949.5. Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. 2015; Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 26(Suppl 5):v56–v68. Erratum in: Ann Oncol 2017;28(Suppl 4):iv167-iv168. DOI: 10.1093/annonc/mdv295. PMID: 26314780.6. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. 2018; Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 24:4846–4861. DOI: 10.3748/wjg.v24.i43.4846. PMID: 30487695. PMCID: PMC6250924.7. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. 2013; Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 310:1473–1481. DOI: 10.1001/jama.2013.279201. PMID: 24104372.8. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. 2017; Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 389:1011–1024. DOI: 10.1016/S0140-6736(16)32409-6. PMID: 28129987.9. Lim KH, Chung E, Khan A, Cao D, Linehan D, Ben-Josef E, et al. 2012; Neoadjuvant therapy of pancreatic cancer: the emerging paradigm? Oncologist. 17:192–200. DOI: 10.1634/theoncologist.2011-0268. PMID: 22250057. PMCID: PMC3286168.10. Tienhoven GV, Versteijne E, Suker M, Groothuis KBC, Busch OR, Bonsing BA, et al. 2018; Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): a randomized, controlled, multicenter phase III trial. J Clin Oncol. 36(18 Suppl):LBA4002. DOI: 10.1200/JCO.2018.36.18_suppl.LBA4002.11. NCCN clinical practice guidelines in oncology - pancretic adenocarcinoma. V1. 2020 [Internet]. Plymouth Meeting: NCCN;2019. cited 2020 Mar 20. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic_blocks.pdf.12. Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, et al. 2015; Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 261:12–17. DOI: 10.1097/SLA.0000000000000867. PMID: 25599322. PMCID: PMC4349683.13. Chun YS, Pawlik TM, Vauthey JN. 2018; 8th edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 25:845–847. DOI: 10.1245/s10434-017-6025-x. PMID: 28752469.14. Tamburrino D, Partelli S, Crippa S, Manzoni A, Maurizi A, Falconi M. 2014; Selection criteria in resectable pancreatic cancer: a biological and morphological approach. World J Gastroenterol. 20:11210–11215. DOI: 10.3748/wjg.v20.i32.11210. PMID: 25170205. PMCID: PMC4145759.15. Bergquist JR, Puig CA, Shubert CR, Groeschl RT, Habermann EB, Kendrick ML, et al. 2016; Carbohydrate antigen 19-9 elevation in anatomically resectable, early stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: a national cancer database study. J Am Coll Surg. 223:52–65. DOI: 10.1016/j.jamcollsurg.2016.02.009. PMID: 27049786.16. Edge SB, Compton CC. 2010; The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. DOI: 10.1245/s10434-010-0985-4. PMID: 20180029.17. Mattiucci GC, Morganti AG, Cellini F, Buwenge M, Casadei R, Farioli A, et al. 2019; Prognostic impact of presurgical CA19-9 level in pancreatic adenocarcinoma: a pooled analysis. Transl Oncol. 12:1–7. DOI: 10.1016/j.tranon.2018.08.017. PMID: 30237099. PMCID: PMC6143718.18. Williams JL, Kadera BE, Nguyen AH, Muthusamy VR, Wainberg ZA, Hines OJ, et al. 2016; CA19-9 normalization during pre-operative treatment predicts longer survival for patients with locally progressed pancreatic cancer. J Gastrointest Surg. 20:1331–1342. DOI: 10.1007/s11605-016-3149-4. PMID: 27114246. PMCID: PMC4919020.19. Spitz FR, Abbruzzese JL, Lee JE, Pisters PW, Lowy AM, Fenoglio CJ, et al. 1997; Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 15:928–937. DOI: 10.1200/JCO.1997.15.3.928. PMID: 9060530.20. Calvo FA, Krengli M, Asencio JM, Serrano J, Poortmans P, Roeder F, et al. 2020; ESTRO IORT Task Force/ACROP recommendations for intraoperative radiation therapy in unresected pancreatic cancer. Radiother Oncol. 148:57–64. DOI: 10.1016/j.radonc.2020.03.040. PMID: 32339779.21. Lambert A, Schwarz L, Borbath I, Henry A, Van Laethem JL, Malka D, et al. 2019; An update on treatment options for pancreatic adenocarcinoma. Ther Adv Med Oncol. 11:1758835919875568. DOI: 10.1177/1758835919875568. PMID: 31598142. PMCID: PMC6763942.22. Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, et al. 2008; Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 299:1019–1026. Erratum in: JAMA 2008;299:1902. DOI: 10.1001/jama.299.9.1019. PMID: 18319412.23. Reni M, Balzano G, Zanon S, Zerbi A, Rimassa L, Castoldi R, et al. 2018; Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol. 3:413–423. DOI: 10.1016/S2468-1253(18)30081-5. PMID: 29625841.24. Heinrich S, Pestalozzi B, Lesurtel M, Berrevoet F, Laurent S, Delpero JR, et al. 2011; Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: a randomized multicenter phase III study (NEOPAC study). BMC Cancer. 11:346. DOI: 10.1186/1471-2407-11-346. PMID: 21831266. PMCID: PMC3176241.25. Hozaeel W, Pauligk C, Homann N, Luley K, Kraus TW, Trojan J, et al. 2015; Randomized multicenter phase II/III study with adjuvant gemcitabine versus neoadjuvant/adjuvant FOLFIRINOX in resectable pancreatic cancer: The NEPAFOX trial. J Clin Oncol. 33(15 Suppl):TPS4152. DOI: 10.1200/jco.2015.33.15_suppl.tps4152.26. Uhl W, Ettrich TJ, Reinacher-Schick AC, Algül H, Friess H, Kornmann M, et al. 2019; NEONAX trial: neoadjuvant plus adjuvant or only adjuvant nab-paclitaxel plus gemcitabine for resectable pancreatic cancer, a phase II study of the AIO pancreatic cancer group (AIO-PAK-0313)- safety interim analysis. J Clin Oncol. 37(15 Suppl):4128. DOI: 10.1200/JCO.2019.37.15_suppl.4128.27. Sohal D, McDonough SL, Ahmad SA, Gandhi N, Beg MS, Wang-Gillam A, et al. 2017; SWOG S1505: a randomized phase II study of perioperative mFOLFIRINOX vs. gemcitabine/nab-paclitaxel as therapy for resectable pancreatic adenocarcinom. J Clin Oncol. 35(4 Suppl):TPS508. DOI: 10.1200/JCO.2017.35.4_suppl.TPS508.28. Raufi AG, Manji GA, Chabot JA, Bates SE. 2019; Neoadjuvant treatment for pancreatic cancer. Semin Oncol. 46:19–27. DOI: 10.1053/j.seminoncol.2018.12.002. PMID: 30630600.29. Valentini V, Calvo F, Reni M, Krempien R, Sedlmayer F, Buchler MW, et al. 2009; Intra-operative radiotherapy (IORT) in pancreatic cancer: joint analysis of the ISIORT-Europe experience. Radiother Oncol. 91:54–59. DOI: 10.1016/j.radonc.2008.07.020. PMID: 18762346.30. Li Y, Feng Q, Jin J, Shi S, Zhang Z, Che X, et al. 2019; Experts' consensus on intraoperative radiotherapy for pancreatic cancer. Cancer Lett. 449:1–7. DOI: 10.1016/j.canlet.2019.01.038. PMID: 30771429.31. Calvo FA, Sole CV, Atahualpa F, Lozano MA, Gomez-Espi M, Calin A, et al. 2013; Chemoradiation for resected pancreatic adenocarcinoma with or without intraoperative radiation therapy boost: long-term outcomes. Pancreatology. 13:576–582. DOI: 10.1016/j.pan.2013.09.002. PMID: 24280572.32. Ogawa K, Ito Y, Karasawa K, Ogawa Y, Onishi H, Kazumoto T, et al. 2010; Patterns of radiotherapy practice for pancreatic cancer in Japan: results of the Japanese Radiation Oncology Study Group (JROSG) survey. Int J Radiat Oncol Biol Phys. 77:743–750. DOI: 10.1016/j.ijrobp.2009.05.063. PMID: 19879060.33. Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, et al. 2022; Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the dutch randomized PREOPANC trial. J Clin Oncol. 40:1220–1230. DOI: 10.1200/JCO.21.02233. PMID: 35084987.34. Taboada AGM, Lominchar PL, Roman LM, García-Alfonso P, Martin AJM, Rodriguez JAB, et al. 2021; Advances in neoadjuvant therapy for resectable pancreatic cancer over the past two decades. Ann Hepatobiliary Pancreat Surg. 25:179–191. DOI: 10.14701/ahbps.2021.25.2.179. PMID: 34053920. PMCID: PMC8180394.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant Therapy for Resectable or Borderline Resectable Pancreatic Cancer
- Role of Neoadjuvant Therapy for Borderline Resectable or Locally Advanced Pancreatic Cancer
- Radiologic Evaluation for Resectability of Pancreatic Adenocarcinoma
- Neoadjuvant and Adjuvant Treatments for Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma: The Current Status of Pancreatic Ductal Adenocarcinoma Treatment in Japan
- Updates of Chemotherapy and Radiotherapy for Pancreatic Cancer